Potassium ferrocyanide trihydrate
PubChem CID
161067
Molecular Formula
Synonyms
- Potassium ferrocyanide trihyrate
- POTASSIUM FERROCYANIDE TRIHYDRATE
- Yellow prussiate
- KALI FERROCYANATUM
- tetrapotassium;iron(2+);hexacyanide;trihydrate
Molecular Weight
422.39 g/mol
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Dates
- Create:2005-08-08
- Modify:2025-01-11
See also:  Potassium Cation (has active moiety).
Potassium Cation (has active moiety).
 Potassium Cation (has active moiety).
Potassium Cation (has active moiety).Chemical Structure Depiction
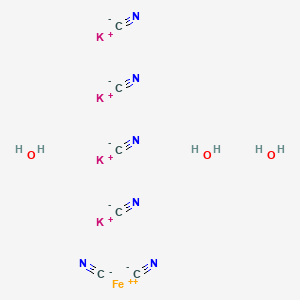
Conformer generation is disallowed since MMFF94s unsupported element, mixture or salt
tetrapotassium;iron(2+);hexacyanide;trihydrate
Computed by Lexichem TK 2.7.0 (PubChem release 2021.10.14)
InChI=1S/6CN.Fe.4K.3H2O/c6*1-2;;;;;;;;/h;;;;;;;;;;;3*1H2/q6*-1;+2;4*+1;;;
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
UTYXJYFJPBYDKY-UHFFFAOYSA-N
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
[C-]#N.[C-]#N.[C-]#N.[C-]#N.[C-]#N.[C-]#N.O.O.O.[K+].[K+].[K+].[K+].[Fe+2]
Computed by OEChem 2.3.0 (PubChem release 2024.12.12)
C6H6FeK4N6O3
Computed by PubChem 2.2 (PubChem release 2021.10.14)
14459-95-1
- Potassium ferrocyanide trihyrate
- POTASSIUM FERROCYANIDE TRIHYDRATE
- Yellow prussiate
- KALI FERROCYANATUM
- tetrapotassium;iron(2+);hexacyanide;trihydrate
- 961WP42S65
- Potassium ferricyanide trihydrate
- UNII-961WP42S65
- Potassium hexacyanoferrate (II) trihydrate
- TETRAPOTASSIUM HEXAKIS(CYANO-C)FERRATE(4-) TRIHYDRATE
- Ferrate(4-), hexakis(cyano-C)-, tetrapotassium, trihydrate, (OC-6-11)-
- KALI FERROCYANATUM [HPUS]
- POTASSIUM FERROCYANIDE TRIHYDRATE [MI]
- Potassium hexacyanoferrate (II) trihydrate ACS grade
- Q47520593
- ?(2)-iron(2+) tetrapotassium hexakis(cyanide) trihydrate
- Potassium hexacyanoferrate (II) trihydrate, Trace metals grade, 99.99%
- POTASSIUM (OC-6-11)-HEXAKIS(CYANO-.KAPPA.C)FERRATE(4-) (4:1) TRIHYDATE
- POTASSIUM (OC-6-11)-HEXAKIS(CYANO-kappaC)FERRATE(4-) (4:1) TRIHYDATE
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
422.39 g/mol
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Donor Count
Property Value
3
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Acceptor Count
Property Value
15
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Rotatable Bond Count
Property Value
0
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Exact Mass
Property Value
421.839900 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Monoisotopic Mass
Property Value
421.839900 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Topological Polar Surface Area
Property Value
146 Ų
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Heavy Atom Count
Property Value
20
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
10
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
14
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2021.10.14)
Slightly efflorescent solid; [Merck Index] Pale yellow odorless crystals; [Alfa Aesar MSDS]
Metals -> Metals, Inorganic Compounds
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Mixtures, Components, and Neutralized Forms Count
Similar Compounds (2D)
Similar Conformers (3D)
Same Count
 Potassium Cation (has active moiety)
Potassium Cation (has active moiety)Homeopathic product and label
Sources/Uses
Used as an oxidizer; [Merck Index] Used in drying colors, tempering steel, dyeing, making explosives, process engraving, and lithography; [Hawley]
Merck Index - O'Neil MJ, Heckelman PE, Dobbelaar PH, Roman KJ (eds). The Merck Index, An Encyclopedia of Chemicals, Drugs, and Biologicals, 15th Ed. Cambridge, UK: The Royal Society of Chemistry, 2013.
Hawley - Lewis RJ. _Hawley's Condensed Chemical Dictionary, _15th Ed. New York: John Wiley & Sons, 2007.
Industrial Processes with risk of exposure
Steel Producing [Category: Industry]
Textiles (Printing, Dyeing, or Finishing) [Category: Industry]
May cause irritation; [Alfa Aesar MSDS] See Potassium ferrocyanide.
- ChemIDplusPotassium ferrocyanide trihydratehttps://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0014459951ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingPOTASSIUM FERROCYANIDE TRIHYDRATEhttps://gsrs.ncats.nih.gov/ginas/app/beta/substances/961WP42S65
- DailyMedPOTASSIUM FERROCYANIDE TRIHYDRATEhttps://dailymed.nlm.nih.gov/dailymed/search.cfm?labeltype=all&query=POTASSIUM+FERROCYANIDE+TRIHYDRATE
- Haz-Map, Information on Hazardous Chemicals and Occupational DiseasesLICENSECopyright (c) 2022 Haz-Map(R). All rights reserved. Unless otherwise indicated, all materials from Haz-Map are copyrighted by Haz-Map(R). No part of these materials, either text or image may be used for any purpose other than for personal use. Therefore, reproduction, modification, storage in a retrieval system or retransmission, in any form or by any means, electronic, mechanical or otherwise, for reasons other than personal use, is strictly prohibited without prior written permission.https://haz-map.com/AboutPotassium ferrocyanide trihydratehttps://haz-map.com/Agents/21566
- National Drug Code (NDC) DirectoryLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingPOTASSIUM FERROCYANIDE TRIHYDRATEhttps://www.fda.gov/drugs/drug-approvals-and-databases/national-drug-code-directory
- Wikidatapotassium ferrocyanide trihydratehttps://www.wikidata.org/wiki/Q47520593
- PubChem
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- NCBI
CONTENTS
 CID 5462222 (Potassium)
CID 5462222 (Potassium) CID 23925 (Iron)
CID 23925 (Iron) CID 768 (Hydrogen Cyanide)
CID 768 (Hydrogen Cyanide) CID 962 (Water)
CID 962 (Water)