Pirlimycin Hydrochloride
PubChem CID
20055247
Molecular Formula
Synonyms
- Pirlimycin HCl
- Pirlimycin hydrochloride hydrate
- Pirlimycin Hydrochloride
- 77495-92-2
- UNII-8S09O559AQ
Molecular Weight
465.4 g/mol
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Parent Compound
Component Compounds
Dates
- Create:2007-12-05
- Modify:2025-01-18
Description
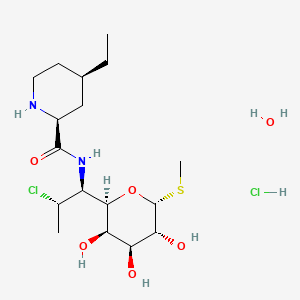
Pirlimycin Hydrochloride is the hydrochloride salt form of pirlimycin, a derivative of clindamycin with a 6-membered ring replacing clindamycin's 5-membered ring. Compared to clindimycin, pirlimycin has increased activity against gram-positive bacteria, including Staphylococcus aureus and coagulase negative species of Staphylococcus and Streptococcus. This agent is primarily used in the treatment of mastitis in cattle.
PIRLIMYCIN HYDROCHLORIDE is a small molecule drug that was first approved in 2001.
See also:  Pirlimycin (has active moiety).
Pirlimycin (has active moiety).
 Pirlimycin (has active moiety).
Pirlimycin (has active moiety).Chemical Structure Depiction
3D Conformer of Parent
(2S,4R)-N-[(1S,2S)-2-chloro-1-[(2R,3R,4S,5R,6R)-3,4,5-trihydroxy-6-methylsulfanyloxan-2-yl]propyl]-4-ethylpiperidine-2-carboxamide;hydrate;hydrochloride
Computed by Lexichem TK 2.7.0 (PubChem release 2021.10.14)
InChI=1S/C17H31ClN2O5S.ClH.H2O/c1-4-9-5-6-19-10(7-9)16(24)20-11(8(2)18)15-13(22)12(21)14(23)17(25-15)26-3;;/h8-15,17,19,21-23H,4-7H2,1-3H3,(H,20,24);1H;1H2/t8-,9+,10-,11+,12-,13+,14+,15+,17+;;/m0../s1
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
CHAZSEMQYSZBFN-RWMVMHIMSA-N
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
CC[C@@H]1CCN[C@@H](C1)C(=O)N[C@@H]([C@@H]2[C@@H]([C@@H]([C@H]([C@H](O2)SC)O)O)O)[C@H](C)Cl.O.Cl
Computed by OEChem 2.3.0 (PubChem release 2024.12.12)
C17H34Cl2N2O6S
Computed by PubChem 2.2 (PubChem release 2021.10.14)
77495-92-2
- cis-4-ethyl-L-picecolic acid amide of 7-deoxy-7(S)chloromethylthio lincosaminide hydrochloride
- pirlimycin
- pirlimycin monohydrochloride monohydrate, (2S-cis)-isomer
- pirlimycin monohydrochloride, (2R-cis)-isomer
- U 57930E
- U-57930E
- Pirlimycin HCl
- Pirlimycin hydrochloride hydrate
- Pirlimycin Hydrochloride
- 77495-92-2
- UNII-8S09O559AQ
- Pirlimycin hydrochloride [USAN]
- U-57,930E
- 8S09O559AQ
- Pirsue
- PIRLIMYCIN HYDROCHLORIDE MONOHYDRATE
- U-57930E
- Pirlimycin hydrochloride (USAN)
- UNII-M5OP0556CW
- Pirlimycin hydrochloride hydrate (JAN)
- PIRLIMYCIN HYDROCHLORIDE (MART.)
- PIRLIMYCIN HYDROCHLORIDE [MART.]
- L-threo-alpha-D-galacto-Octopyranoside, methyl 7-chloro-6,7,8-trideoxy-6-(((4-ethyl-2-piperidinyl)carbonyl)amino)-1-thio-, monohydrochloride, monohydrate, (2S-cis)-
- PIRLIMYCIN HYDROCHLORIDE HYDRATE [JAN]
- PIRLIMYCIN HYDROCHLORIDE ANHYDROUS
- U 57930E
- Pirsue Sterile Solution
- L-threo-alpha-D-galacto-Octopyranoside, methyl 7-chloro-6,7,8-trideoxy-6-((((2S,4R)-4-ethyl-2-piperidinyl)carbonyl)amino)-1-thio-, monohydrochloride
- Pirlimycinhydrochloridehydrate
- SCHEMBL193725
- CHEMBL3989899
- Methyl 7-chloro-6,7,8-trideoxy-6-cis-(4-ethyl-L-pipecolamido)-1-thio-L-threo-alpha-D-galacto-octopyranoside monohydrochloride monohydrate
- PIRLIMYCIN HYDROCHLORIDE [GREEN BOOK]
- D05501
- Q27270945
- (2S,4R)-N-[(1S,2S)-2-chloro-1-[(2R,3R,4S,5R,6R)-3,4,5-trihydroxy-6-methylsulfanyloxan-2-yl]propyl]-4-ethylpiperidine-2-carboxamide;hydrate;hydrochloride
- L-THREO-.ALPHA.-D-GALACTO-OCTOPYRANOSIDE, METHYL 7-CHLORO-6,7,8-TRIDEOXY-6-(((4-ETHYL-2-PIPERIDINYL)CARBONYL)AMINO)-1-THIO-, MONOHYDROCHLORIDE, MONOHYDRATE, (2S-CIS)-
- L-THREO-alpha-D-GALACTO-OCTOPYRANOSIDE, METHYL 7-CHLORO-6,7,8-TRIDEOXY-6-((((2S,4R)-4-ETHYL-2-PIPERIDINYL)CARBONYL)AMINO)-1-THIO-, HYDROCHLORIDE (1:1)
- METHYL 7-CHLORO-6,7,8-TRIDEOXY-6-CIS-4-ETHYL-L-PIPECOLAMIDO)-1-THIO-L-THREO-.ALPHA.-D-GALACTO-OCTOPYRANOSIDE MONOHYDROCHLORIDE MONOHYDRATE
- METHYL 7-CHLORO-6,7,8-TRIDEOXY-6-CIS-4-ETHYL-L-PIPECOLAMIDO)-1-THIO-L-THREO-alpha-D-GALACTO-OCTOPYRANOSIDE MONOHYDROCHLORIDE
- METHYL 7-CHLORO-6,7,8-TRIDEOXY-6-CIS-4-ETHYL-L-PIPECOLAMIDO)-1-THIO-L-THREO-alpha-D-GALACTO-OCTOPYRANOSIDE MONOHYDROCHLORIDE MONOHYDRATE
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
465.4 g/mol
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Donor Count
Property Value
7
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Acceptor Count
Property Value
8
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Rotatable Bond Count
Property Value
6
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Exact Mass
Property Value
464.1514634 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Monoisotopic Mass
Property Value
464.1514634 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Topological Polar Surface Area
Property Value
137 Ų
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Heavy Atom Count
Property Value
28
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
474
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
9
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
3
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2021.10.14)
Veterinary drugs -> Antibacterials for intramammary use -> Veterinary pharmacotherapeutic group -> EMA Drug Category
Active Ingredients (Pirlimycin Hydrochloride) -> FDA Greenbook
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Same Connectivity Count
Same Parent, Connectivity Count
Same Parent, Isotope Count
Same Parent, Exact Count
Mixtures, Components, and Neutralized Forms Count
Similar Compounds (2D)
Similar Conformers (3D)
Same Count
 Pirlimycin (has active moiety)
Pirlimycin (has active moiety)For the treatment of subclinical mastitis in lactating cows due to Gram-positive cocci susceptible to pirlimycin including staphylococcal organisms such as Staphylococcus aureus, both penicillinase-positive and penicillinase-negative, and coagulase-negative staphylococci; streptococcal organisms including Streptococcus agalactiae, Streptococcus dysgalactiae and Streptococcus uberis.
Medicine
Category
Veterinary drugs
Active Substance
pirlimycin
INN/Common name
pirlimycin
Pharmacotherapeutic Classes
Antibacterials for intramammary use
Status
This medicine is authorized for use in the European Union
Company
Zoetis Belgium SA
Market Date
2001-01-29
JECFA Functional Classes
Veterinary Drug -> ANTIMICROBIAL_AGENT;
Chemical Name
PIRSUE STERILE SOLUTION
Evaluation Year
2004
ADI
0–8 µg/kg bw/d
Comments
The Committee noted that pirlimycin belongs to the class of lincosamide antibiotics that are known to be associated with perturbation of the gastrointestinal microflora following administration in humans. The ADI based on MIC data was considered to be extremely conservative considering the findings in a hamster model of pseudomembranous colitis and in humans. Therefore, the Committee established a microbiological ADI of 0–8 µg/kg bw/d on the basis of the NOEL for gastrointestinal effects in humans of 50 mg (0.83 mg/kg bw for a 60-kg adult) and a safety factor of 100, rounded to one significant figure.
Report
Tox Monograph
QJ51FF90
Veterinary drugs -> Antibacterials for intramammary use -> Veterinary pharmacotherapeutic group -> EMA Drug Category
Animal Drugs -> FDA Approved Animal Drug Products (Green Book) -> Active Ingredients
Veterinary Drug -> ANTIMICROBIAL_AGENT; -> JECFA Functional Classes
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=CHAZSEMQYSZBFN-RWMVMHIMSA-N
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.html
- ChemIDplusPirlimycin hydrochloride [USAN]https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0077495922ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingPIRLIMYCIN HYDROCHLORIDEhttps://gsrs.ncats.nih.gov/ginas/app/beta/substances/8S09O559AQ
- European Medicines Agency (EMA)LICENSEInformation on the European Medicines Agency's (EMA) website is subject to a disclaimer and copyright and limited reproduction notices.https://www.ema.europa.eu/en/about-us/legal-noticePirsue (EMEA/V/C/000054)https://www.ema.europa.eu/en/medicines/veterinary/EPAR/pirsue
- FDA Approved Animal Drug Products (Green Book)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingPirlimycin Hydrochloridehttps://www.fda.gov/animal-veterinary/products/approved-animal-drug-products-green-book
- Joint FAO/WHO Expert Committee on Food Additives (JECFA)LICENSEPermission from WHO is not required for the use of WHO materials issued under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Intergovernmental Organization (CC BY-NC-SA 3.0 IGO) licence.https://www.who.int/about/policies/publishing/copyrightPIRSUE STERILE SOLUTIONhttps://apps.who.int/food-additives-contaminants-jecfa-database/Home/Chemical/5002
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.htmlAntiinfectiveshttp://www.genome.jp/kegg-bin/get_htext?br08307.keg
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- Open TargetsLICENSEDatasets generated by the Open Targets Platform are freely available for download.https://platform-docs.opentargets.org/licencePIRLIMYCIN HYDROCHLORIDEhttps://platform.opentargets.org/drug/CHEMBL3989899
- Springer Nature
- Wikidatapirlimycin hydrochloride hydratehttps://www.wikidata.org/wiki/Q27270945
- PubChem
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.html
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 389158133https://pubchem.ncbi.nlm.nih.gov/substance/389158133
CONTENTS

 CID 313 (Hydrochloric Acid)
CID 313 (Hydrochloric Acid) CID 962 (Water)
CID 962 (Water)