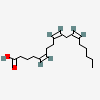
Pinolenic acid
PubChem CID
5312495
Molecular Formula
Synonyms
- Pinolenic acid
- 16833-54-8
- (5Z,9Z,12Z)-octadeca-5,9,12-trienoic acid
- 5Z,9Z,12Z-octadecatrienoic acid
- Pinolenic acid [MI]
Molecular Weight
278.4 g/mol
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Dates
- Create:2006-01-13
- Modify:2025-01-04
Description
(5Z,9Z,12Z)-octadecatrienoic acid is an octadecatrienoic acid having three double bonds located at positions 5, 9 and 12 (the all-cis-isomer). It has a role as a plant metabolite and an antineoplastic agent.
Pinolenic acid has been reported in Teucrium cubense and Pinus koraiensis with data available.
Chemical Structure Depiction

(5Z,9Z,12Z)-octadeca-5,9,12-trienoic acid
Computed by Lexichem TK 2.7.0 (PubChem release 2021.10.14)
InChI=1S/C18H30O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18(19)20/h6-7,9-10,13-14H,2-5,8,11-12,15-17H2,1H3,(H,19,20)/b7-6-,10-9-,14-13-
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
HXQHFNIKBKZGRP-URPRIDOGSA-N
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
CCCCC/C=C\C/C=C\CC/C=C\CCCC(=O)O
Computed by OEChem 2.3.0 (PubChem release 2024.12.12)
C18H30O2
Computed by PubChem 2.2 (PubChem release 2021.10.14)
- 5,9,12-octadecatrienoic acid
- 5,9,12-octadecatrienoic acid, (cis)-isomer
- 5,9,12-octadecatrienoic acid, (Z,Z,E)-isomer
- columbinic acid
- pinolenic acid
- trans-5,9,12-octadecatrienoic acid
- Pinolenic acid
- 16833-54-8
- (5Z,9Z,12Z)-octadeca-5,9,12-trienoic acid
- 5Z,9Z,12Z-octadecatrienoic acid
- Pinolenic acid [MI]
- cis,cis,cis-5,9,12-Octadecatrienoic acid
- B810C93MD7
- 5,9,12-Octadecatrienoic acid, (5Z,9Z,12Z)-
- C18:3n-6,9,13
- UNII-B810C93MD7
- all-cis-octadeca-5,9,12-trienoic acid
- (z,z,z)-5,9,12-octadecatrienoic acid
- SCHEMBL1021463
- CHEBI:86136
- DTXSID00895852
- HXQHFNIKBKZGRP-URPRIDOGSA-N
- HMS3650G16
- LMFA01030344
- (5Z,9Z,12Z)-octadecatrienoic acid
- AKOS040754743
- 1ST164627
- 5,9,12-OCTADECATRIENOIC ACID, CIS
- HY-119546
- CS-0068703
- SR-01000946285
- J-010448
- SR-01000946285-1
- Q63408763
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
278.4 g/mol
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
XLogP3-AA
Property Value
5.6
Reference
Computed by XLogP3 3.0 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Donor Count
Property Value
1
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Acceptor Count
Property Value
2
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Rotatable Bond Count
Property Value
13
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Exact Mass
Property Value
278.224580195 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Monoisotopic Mass
Property Value
278.224580195 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Topological Polar Surface Area
Property Value
37.3Ų
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Heavy Atom Count
Property Value
20
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
301
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
3
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
1
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2021.10.14)
Potential endocrine disrupting compound
S109 | PARCEDC | List of 7074 potential endocrine disrupting compounds (EDCs) by PARC T4.2 | DOI:10.5281/zenodo.10944198
Fatty Acyls [FA] -> Fatty Acids and Conjugates [FA01] -> Unsaturated fatty acids [FA0103]
NIST Number
1208104
Instrument Type
IT/ion trap
Collision Energy
0
Spectrum Type
MS2
Precursor Type
[M-H]-
Precursor m/z
277.2173
Total Peaks
30
m/z Top Peak
233.2
m/z 2nd Highest
259.2
m/z 3rd Highest
275.2
Thumbnail
NIST Number
1212928
Instrument Type
IT/ion trap
Collision Energy
0
Spectrum Type
MS2
Precursor Type
[M+H]+
Precursor m/z
279.2319
Total Peaks
74
m/z Top Peak
261.3
m/z 2nd Highest
243.3
m/z 3rd Highest
181.1
Thumbnail
Accession ID
Authors
Plant Biology, The Noble Foundation, Ardmore, OK, US/Dennis Fine, Daniel Wherritt, and Lloyd Sumner
Instrument
Bruker impact HD
Instrument Type
LC-ESI-QTOF
MS Level
MS2
Ionization Mode
NEGATIVE
Ionization
ESI
Collision Energy
10 eV
Column Name
Waters Acquity BEH C18 1.7um x 2.1 x 150 mm
Retention Time
1705.8 sec
Precursor m/z
277.2183
Precursor Adduct
[M-H]-
Top 5 Peaks
277.2183 999
278.2213 152
License
CC BY-NC-SA
Accession ID
Authors
Plant Biology, The Noble Foundation, Ardmore, OK, US/Dennis Fine, Daniel Wherritt, and Lloyd Sumner
Instrument
Bruker impact HD
Instrument Type
LC-ESI-QTOF
MS Level
MS
Ionization Mode
NEGATIVE
Ionization
ESI
Column Name
Waters Acquity BEH C18 1.7um x 2.1 x 150 mm
Retention Time
1705.8 sec
Top 5 Peaks
277.2182 999
278.2213 184
345.2055 116
556.0034 90
577.426 66
License
CC BY-NC-SA
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Same Connectivity Count
Same Isotope Count
Same Parent, Connectivity Count
Same Parent, Isotope Count
Same Parent, Exact Count
Mixtures, Components, and Neutralized Forms Count
Similar Compounds (2D)
Similar Conformers (3D)
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=HXQHFNIKBKZGRP-URPRIDOGSA-N
The LOTUS Initiative for Open Natural Products Research: frozen dataset union wikidata (with metadata) | DOI:10.5281/zenodo.5794106
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/Pinolenic acidhttps://commonchemistry.cas.org/detail?cas_rn=16833-54-8
- ChemIDplusChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA DSSToxcis,cis,cis-5,9,12-Octadecatrienoic acidhttps://comptox.epa.gov/dashboard/DTXSID00895852CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- ChEBI(5Z,9Z,12Z)-octadecatrienoic acidhttps://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:86136
- LOTUS - the natural products occurrence databaseLICENSEThe code for LOTUS is released under the GNU General Public License v3.0.https://lotus.nprod.net/Pinolenic acidhttps://www.wikidata.org/wiki/Q2823302LOTUS Treehttps://lotus.naturalproducts.net/
- KNApSAcK Species-Metabolite DatabaseOctadeca-5,9,12-trienoic acidhttp://www.knapsackfamily.com/knapsack_core/info.php?sname=C_ID&word=C00053586
- Natural Product Activity and Species Source (NPASS)
- LIPID MAPSLipid Classificationhttps://www.lipidmaps.org/
- MassBank Europe
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- Metabolomics Workbench
- NIST Mass Spectrometry Data CenterLICENSEData covered by the Standard Reference Data Act of 1968 as amended.https://www.nist.gov/srd/public-lawPinolenic acidhttp://www.nist.gov/srd/nist1a.cfm
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/
- Springer Nature
- Wikidatapinolenic acidhttps://www.wikidata.org/wiki/Q2823302
- WikipediaPinolenic acidhttps://en.wikipedia.org/wiki/Pinolenic_acid
- PubChem
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.html5,9,12-octadecatrienoic acidhttps://www.ncbi.nlm.nih.gov/mesh/67034302
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 391103029https://pubchem.ncbi.nlm.nih.gov/substance/391103029
CONTENTS