Ovral
PubChem CID
24706
Molecular Formula
Synonyms
- ethinylestradiol & levonorgestrel
- ethinylestradiol + levonorgestrel
- ethinylestradiol / levonorgestrel
- ethinylestradiol and levonorgestrel
- Ovral
Molecular Weight
608.8 g/mol
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Component Compounds
Dates
- Create:2005-08-08
- Modify:2025-02-01
Description
Ethinyl Estradiol/Levonorgestrel is a combination of two steroid sex hormones, an estrogen and a progestin, used for contraceptive purposes. Estradiol, the endogenous counterpart of ethinyl estradiol (EE), is the principal, most potent estrogen hormone produced by the ovaries and is vital to the maintenance of fertility and secondary sexual characteristics in females. Levonorgestrel is a synthetic progestogen. This drug combination prevents or delays ovulation and causes a variety of hormonal changes. Ethinyl estradiol inhibits the release of follicle stimulating hormone (FSH), thus suppressing the development of ovarian follicle; levonorgestrel inhibits the release of luteinizing hormone (LH), thus preventing ovulation. This combination of agents alters the endometrium in such a way as to discourage implantation. (NCI04)
See also: Ethinyl estradiol; norgestrel (annotation moved to); Norgestrel and Ethinyl Estradiol (annotation moved to).
Chemical Structure Depiction
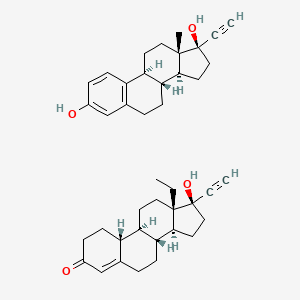
Conformer generation is disallowed since mixture or salt
(8R,9S,10R,13S,14S,17R)-13-ethyl-17-ethynyl-17-hydroxy-1,2,6,7,8,9,10,11,12,14,15,16-dodecahydrocyclopenta[a]phenanthren-3-one;(8R,9S,13S,14S,17R)-17-ethynyl-13-methyl-7,8,9,11,12,14,15,16-octahydro-6H-cyclopenta[a]phenanthrene-3,17-diol
Computed by Lexichem TK 2.7.0 (PubChem release 2021.10.14)
InChI=1S/C21H28O2.C20H24O2/c1-3-20-11-9-17-16-8-6-15(22)13-14(16)5-7-18(17)19(20)10-12-21(20,23)4-2;1-3-20(22)11-9-18-17-6-4-13-12-14(21)5-7-15(13)16(17)8-10-19(18,20)2/h2,13,16-19,23H,3,5-12H2,1H3;1,5,7,12,16-18,21-22H,4,6,8-11H2,2H3/t16-,17+,18+,19-,20-,21-;16-,17-,18+,19+,20+/m01/s1
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
ORKBYCQJWQBPFG-WOMZHKBXSA-N
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
CC[C@]12CC[C@H]3[C@H]([C@@H]1CC[C@]2(C#C)O)CCC4=CC(=O)CC[C@H]34.C[C@]12CC[C@H]3[C@H]([C@@H]1CC[C@]2(C#C)O)CCC4=C3C=CC(=C4)O
Computed by OEChem 2.3.0 (PubChem release 2024.12.12)
C41H52O4
Computed by PubChem 2.2 (PubChem release 2021.10.14)
70208-30-9, 8063-84-1, 8064-50-4
110541-57-6, 37263-36-8, 52232-43-6, 55927-62-3
- ethinylestradiol & levonorgestrel
- ethinylestradiol + levonorgestrel
- ethinylestradiol / levonorgestrel
- ethinylestradiol and levonorgestrel
- Ovral
- Microgynon
- 39366-37-5
- Trikvilar
- Elifemme
- 8056-51-7
- Minisiston
- Trinordiol
- Anteovin
- Eugynon
- Gravistat
- Rigevidon
- Sequostat
- Stediril
- Trigynon
- Triquilar
- Trisiston
- Adepal
- Ovidon
- Biphasil
- Duoluton
- Femenal
- Follimin
- Follinett
- Follinyl
- Gynatrol
- Logynon
- Minidril
- Minigynon
- Neogynon
- Neovlar
- Neovletta
- Nordiol
- Orasecron
- Ovranett
- Primovlar
- Quasense
- Sequilar
- Sequilarum
- Alesse
- Ediwal
- Levora
- Lybrel
- Ovran
- Anna
- Low-ogestrel
- Pro-Duosterone
- Lo-Femenal
- Stediril D
- Tri-Regol
- Microgynon 30
- Ovral L
- Microvlar 30
- Triphasil-21
- Biphasil 21
- Biphasil 28
- Stederil 30
- Schering PC 4
- Eugynon 30
- Nordiol-28
- Alesse 28
- LEVLITE
- Ovral 21
- Ovral 28
- aviane
- Loseasonique
- Altavera
- Introvale
- Jolessa
- Levonest
- Marlissa
- PC 4 (contraceptive)
- Quartette
- Portia
- Sronyx
- Norgestrel and Ethinyl Estradiol
- Norgestrel-ethinyl estradiol
- (8R,9S,10R,13S,14S,17R)-13-ethyl-17-ethynyl-17-hydroxy-1,2,6,7,8,9,10,11,12,14,15,16-dodecahydrocyclopenta[a]phenanthren-3-one;(8R,9S,13S,14S,17R)-17-ethynyl-13-methyl-7,8,9,11,12,14,15,16-octahydro-6H-cyclopenta[a]phenanthrene-3,17-diol
- LOC 31A
- Aviane-21Fe
- SHB 261AB
- SHB 264AB
- CRYSELLE
- CCRIS 7261
- Ethinyl estradiol/levonorgestrel
- Levonorgestrel/ethinyl estradiol
- LOC 31
- WL 33
- WY-E 104
- TRIPHASIL-28
- ETHINYL ESTRADIOL-NORGESTREL COMBINATION
- Levonorgestrel and Ethinyl Estradiol
- ENPRESSE-21
- ENPRESSE-28
- LOW-OGESTREL-21
- LOW-OGESTREL-28
- NORDETTE-21
- NORDETTE-28
- LESSINA-21
- LESSINA-28
- TRIVORA-21
- TRIVORA-28
- AVIANE-21
- AVIANE-28
- PORTIA-21
- PORTIA-28
- LO/OVRAL-28
- OVRAL-28
- aless
- SH 71121
- Twirla
- Vienva
- Levonorgestrel, ethinyl estradiol
- Norgestrel-ethynylestradiol mixt.
- SH D00264A
- OGESTREL 0.5/50-21
- OGESTREL 0.5/50-28
- Levonorgestrel-ethinyl estradiol mixt.
- LEVORA 0.15/30-21
- LEVORA 0.15/30-28
- Ethinylestradiol - levonorgestrel mixt.
- Levonorgestrel - ethynylestradiol mixt.
- PREVEN EMERGENCY CONTRACEPTIVE KIT
- AG 200-15
- Levonorgestrel and Ethinyl Estradiol tablets
- SCHEMBL4451192
- DTXSID90192569
- AKOS040751734
- 18,19-Dinorpregn-4-en-20-yn-3-one, 13-ethyl-17-hydroxy-, (17-alpha)-(+-)-, mixt. with (17-alpha)-19-norpregna-1,3,5(10)-trien-20-yne-3,17-diol
- 18,19-Dinorpregn-4-en-20-yn-3-one, 13-ethyl-17-hydroxy-, (17-alpha)-, mixt. with (17-alpha)-19-norpregna-1,3,5(10)-trien-20-yne-3,17-diol
- AG200-15
- ethinyl estradiol, levonorgestrel drug combination
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
608.8 g/mol
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Donor Count
Property Value
3
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Acceptor Count
Property Value
4
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Rotatable Bond Count
Property Value
3
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Exact Mass
Property Value
608.38656014 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Monoisotopic Mass
Property Value
608.38656014 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Topological Polar Surface Area
Property Value
77.8 Ų
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Heavy Atom Count
Property Value
45
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
1110
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
11
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
2
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2021.10.14)
Human drug -> Discontinued
Human drug -> Prescription
Human drug -> Prescription; Discontinued
Oral hormonal contraceptives
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Same Connectivity Count
Mixtures, Components, and Neutralized Forms Count
Similar Compounds (2D)
Similar Conformers (3D)
Same Count
- Ethinyl estradiol; norgestrel (annotation moved to)
- Norgestrel and Ethinyl Estradiol (annotation moved to)
Drug
Drug Classes
Formulation
Indication
Drug
Drug Classes
Oral hormonal contraceptives
Formulation
(1) Oral - Solid: 50 µg + 250 µg [4]; (2) Oral - Solid: 30 µg + 150 µg
Indication
(1) Contact with health services for postcoital contraception; (2) Contact with health services for contraceptive management
Drug and label
Drug and label
Drug and label
G03AA07
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=ORKBYCQJWQBPFG-WOMZHKBXSA-N
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/Ethinylestradiol-levonorgestrel mixt.https://commonchemistry.cas.org/detail?cas_rn=39366-37-518,19-Dinorpregn-4-en-20-yn-3-one, 13-ethyl-17-hydroxy-, (17α)-(±)-, mixt. with (17α)-19-norpregna-1,3,5(10)-trien-20-yne-3,17-diolhttps://commonchemistry.cas.org/detail?cas_rn=8056-51-7
- ChemIDplusEthinyl estradiol mixture with norgestrelhttps://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0008056517Ethinylestradiol mixture with Levonorgestrelhttps://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0039366375ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA DSSToxEthinylestradiol mixture with Levonorgestrelhttps://comptox.epa.gov/dashboard/DTXSID90192569CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- ClinicalTrials.govLICENSEThe ClinicalTrials.gov data carry an international copyright outside the United States and its Territories or Possessions. Some ClinicalTrials.gov data may be subject to the copyright of third parties; you should consult these entities for any additional terms of use.https://clinicaltrials.gov/ct2/about-site/terms-conditions#Use
- Comparative Toxicogenomics Database (CTD)LICENSEIt is to be used only for research and educational purposes. Any reproduction or use for commercial purpose is prohibited without the prior express written permission of NC State University.http://ctdbase.org/about/legal.jspEthinyl Estradiol-Norgestrel Combinationhttps://ctdbase.org/detail.go?type=chem&acc=D019304
- DailyMedLEVONORGESTREL/ETHINYL ESTRADIOLhttps://dailymed.nlm.nih.gov/dailymed/search.cfm?labeltype=all&query=LEVONORGESTREL/ETHINYL+ESTRADIOLLEVONORGESTREL AND ETHINYL ESTRADIOL TABLETShttps://dailymed.nlm.nih.gov/dailymed/search.cfm?labeltype=all&query=LEVONORGESTREL+AND+ETHINYL+ESTRADIOL+TABLETSLEVONORGESTREL AND ETHINYL ESTRADIOLhttps://dailymed.nlm.nih.gov/dailymed/search.cfm?labeltype=all&query=LEVONORGESTREL+AND+ETHINYL+ESTRADIOL
- Drugs@FDALICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingTRIPHASIL-21https://www.accessdata.fda.gov/scripts/cder/daf/
- WHO Model Lists of Essential MedicinesLICENSEPermission from WHO is not required for the use of WHO materials issued under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Intergovernmental Organization (CC BY-NC-SA 3.0 IGO) license.https://www.who.int/about/policies/publishing/copyrightEthinylestradiol + levonorgestrelhttps://list.essentialmeds.org/medicines/184
- EU Clinical Trials Register
- National Drug Code (NDC) DirectoryLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingLEVONORGESTREL/ETHINYL ESTRADIOLhttps://www.fda.gov/drugs/drug-approvals-and-databases/national-drug-code-directory
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuse
- Springer Nature
- WikidataEthinylestradiol mixture with Levonorgestrelhttps://www.wikidata.org/wiki/Q83065240
- PubChem
- PATENTSCOPE (WIPO)SID 388679567https://pubchem.ncbi.nlm.nih.gov/substance/388679567
- NCBI
CONTENTS

 CID 13109 (Norgestrel)
CID 13109 (Norgestrel) CID 5991 (Ethinyl Estradiol)
CID 5991 (Ethinyl Estradiol)