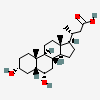
Norhyodeoxycholic acid
PubChem CID
70676190
Molecular Formula
Synonyms
- Norhyodeoxycholic acid
- 77518-23-1
- (3R)-3-[(3R,5R,6S,8S,9S,10R,13R,14S,17R)-3,6-dihydroxy-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl]butanoic acid
- (5beta)-3alpha,6alpha-dihydroxy-24-norcholan-23-oic acid
- 24-nor-3alpha,6alpha-dihydroxy-5beta-cholan-23-oic acid
Molecular Weight
378.5 g/mol
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Dates
- Create:2012-12-03
- Modify:2025-01-18
Description
Norhyodeoxycholic acid is a cholanoid.
Chemical Structure Depiction
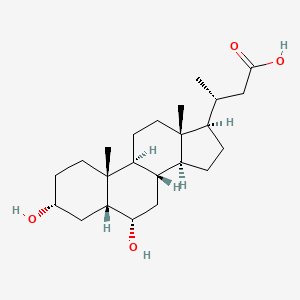
(3R)-3-[(3R,5R,6S,8S,9S,10R,13R,14S,17R)-3,6-dihydroxy-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl]butanoic acid
Computed by Lexichem TK 2.7.0 (PubChem release 2021.05.07)
InChI=1S/C23H38O4/c1-13(10-21(26)27)16-4-5-17-15-12-20(25)19-11-14(24)6-8-23(19,3)18(15)7-9-22(16,17)2/h13-20,24-25H,4-12H2,1-3H3,(H,26,27)/t13-,14-,15+,16-,17+,18+,19+,20+,22-,23-/m1/s1
Computed by InChI 1.0.6 (PubChem release 2021.05.07)
ZBAVIUQLFUYWMT-NNUWNQTCSA-N
Computed by InChI 1.0.6 (PubChem release 2021.05.07)
C[C@H](CC(=O)O)[C@H]1CC[C@@H]2[C@@]1(CC[C@H]3[C@H]2C[C@@H]([C@H]4[C@@]3(CC[C@H](C4)O)C)O)C
Computed by OEChem 2.3.0 (PubChem release 2024.12.12)
C23H38O4
Computed by PubChem 2.1 (PubChem release 2021.05.07)
- Norhyodeoxycholic acid
- 77518-23-1
- (3R)-3-[(3R,5R,6S,8S,9S,10R,13R,14S,17R)-3,6-dihydroxy-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl]butanoic acid
- (5beta)-3alpha,6alpha-dihydroxy-24-norcholan-23-oic acid
- 24-nor-3alpha,6alpha-dihydroxy-5beta-cholan-23-oic acid
- SCHEMBL23695296
- CHEBI:181188
- LMST04060024
- AKOS040755665
- "(3R)-3-((3R,5R,6S,8S,9S,10R,13R,17R)-3,6-dihydroxy-10,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)butanoic acid"
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
378.5 g/mol
Reference
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Property Name
XLogP3-AA
Property Value
4.6
Reference
Computed by XLogP3 3.0 (PubChem release 2021.05.07)
Property Name
Hydrogen Bond Donor Count
Property Value
3
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.05.07)
Property Name
Hydrogen Bond Acceptor Count
Property Value
4
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.05.07)
Property Name
Rotatable Bond Count
Property Value
3
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.05.07)
Property Name
Exact Mass
Property Value
378.27700969 Da
Reference
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Property Name
Monoisotopic Mass
Property Value
378.27700969 Da
Reference
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Property Name
Topological Polar Surface Area
Property Value
77.8 Ų
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.05.07)
Property Name
Heavy Atom Count
Property Value
27
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
591
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.05.07)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
10
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
1
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2012.11.26)
Lipids -> Sterol Lipids [ST] -> Bile acids and derivatives [ST04] -> C23 bile acids, alcohols, and derivatives [ST0406]
MoNA ID
MS Category
Experimental
MS Type
Other
MS Level
MS2
Precursor Type
[2M+H]+
Precursor m/z
757.561
Instrument
qTof
Ionization Mode
positive
Top 5 Peaks
343.262878 100
344.265015 92.50
325.251068 42.14
161.132065 29.33
361.271881 19.93
MoNA ID
MS Category
Experimental
MS Type
Other
MS Level
MS2
Precursor Type
[2M+Na]+
Precursor m/z
779.543
Instrument
qTof
Ionization Mode
positive
Top 5 Peaks
401.263397 100
402.267578 33.92
343.261475 21.30
419.274750 16.14
344.265167 7.67
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Same Connectivity Count
Same Parent, Connectivity Count
Similar Compounds (2D)
Similar Conformers (3D)
Same Count
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=ZBAVIUQLFUYWMT-NNUWNQTCSA-N
- ChEBINorhyodeoxycholic acidhttps://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:181188
- Japan Chemical Substance Dictionary (Nikkaji)
- LIPID MAPSLipid Classificationhttps://www.lipidmaps.org/
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license"(3R)-3-((3R,5R,6S,8S,9S,10R,13R,17R)-3,6-dihydroxy-10,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)butanoic acid"https://mona.fiehnlab.ucdavis.edu/spectra/browse?query=exists(compound.metaData.name:%27InChIKey%27%20and%20compound.metaData.value:%27ZBAVIUQLFUYWMT-NNUWNQTCSA-N%27)
- Metabolomics WorkbenchNorhyodeoxycholic acidhttps://www.metabolomicsworkbench.org/data/StructureData.php?RegNo=72047
- Wikidata24-nor-3alpha,6alpha-dihydroxy-5beta-cholan-23-oic acidhttps://www.wikidata.org/wiki/Q76755691
- PubChem
- PATENTSCOPE (WIPO)SID 481846881https://pubchem.ncbi.nlm.nih.gov/substance/481846881
CONTENTS