Miocamycin
PubChem CID
5282188
Chemical Safety
Molecular Formula
Synonyms
- Miocamycin
- midecamycin acetate
- Acecamycin
- Miokamycin
- Ponsinomycin
Molecular Weight
898.0 g/mol
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Dates
- Create:2005-06-24
- Modify:2025-01-11
Description
Miocamycin is a macrolide type antimicrobial. This drug may be marketed in Japan for clinical use as it is listed in the Japanese pharmacopeia. It has shown to be effective against several gram-positive and gram-negative microbes and may be useful in the treatment of upper and lower respiratory tract infections, and urogenital tract infections or as an alternative to erythromycin treatment.
MIOCAMYCIN is a small molecule drug and has 1 investigational indication.
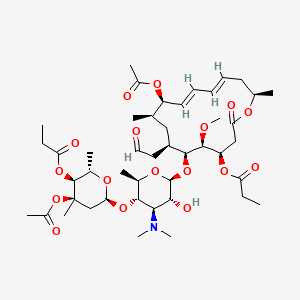
Chemical Structure Depiction
Conformer generation is disallowed since too many atoms, too flexible
[(4R,5S,6S,7R,9R,10R,11E,13E,16R)-10-acetyloxy-6-[(2S,3R,4R,5S,6R)-5-[(2S,4R,5S,6S)-4-acetyloxy-4,6-dimethyl-5-propanoyloxyoxan-2-yl]oxy-4-(dimethylamino)-3-hydroxy-6-methyloxan-2-yl]oxy-5-methoxy-9,16-dimethyl-2-oxo-7-(2-oxoethyl)-1-oxacyclohexadeca-11,13-dien-4-yl] propanoate
Computed by Lexichem TK 2.7.0 (PubChem release 2021.10.14)
InChI=1S/C45H71NO17/c1-13-34(50)59-33-23-36(52)55-26(4)18-16-15-17-19-32(58-29(7)48)25(3)22-31(20-21-47)41(42(33)54-12)62-44-39(53)38(46(10)11)40(27(5)57-44)61-37-24-45(9,63-30(8)49)43(28(6)56-37)60-35(51)14-2/h15-17,19,21,25-28,31-33,37-44,53H,13-14,18,20,22-24H2,1-12H3/b16-15+,19-17+/t25-,26-,27-,28+,31+,32+,33-,37+,38-,39-,40-,41+,42+,43+,44+,45-/m1/s1
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
GQNZGCARKRHPOH-RQIKCTSVSA-N
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
CCC(=O)O[C@@H]1CC(=O)O[C@@H](C/C=C/C=C/[C@@H]([C@@H](C[C@@H]([C@@H]([C@H]1OC)O[C@H]2[C@@H]([C@H]([C@@H]([C@H](O2)C)O[C@H]3C[C@@]([C@H]([C@@H](O3)C)OC(=O)CC)(C)OC(=O)C)N(C)C)O)CC=O)C)OC(=O)C)C
Computed by OEChem 2.3.0 (PubChem release 2024.12.12)
C45H71NO17
Computed by PubChem 2.2 (PubChem release 2021.10.14)
- maidimeisu
- Midecamin
- midecamycin
- midecamycin acetate
- midecamycin diacetate
- midekamycin
- midekamycin acetate
- Mosil
- mydecamycin
- Myoxam
- neoisomidecamycin
- Normicina
- SF 837
- SF-837
- Miocamycin
- midecamycin acetate
- Acecamycin
- Miokamycin
- Ponsinomycin
- 55881-07-7
- 3'',9-Diacetylmydecamycin
- Midecamycin acetate [JAN]
- Miocamycin (TN)
- EINECS 259-879-6
- UNII-3T48CPS7U2
- midecamycin diacetate
- Leucomycin V, 3B,9-diacetate 3,4B-dipropanoate
- 9,3''-Di-O-Acetylmidecamycin
- [(4R,5S,6S,7R,9R,10R,11E,13E,16R)-10-acetyloxy-6-[(2S,3R,4R,5S,6R)-5-[(2S,4R,5S,6S)-4-acetyloxy-4,6-dimethyl-5-propanoyloxyoxan-2-yl]oxy-4-(dimethylamino)-3-hydroxy-6-methyloxan-2-yl]oxy-5-methoxy-9,16-dimethyl-2-oxo-7-(2-oxoethyl)-1-oxacyclohexadeca-11,13-dien-4-yl] propanoate
- MIOCAMYCIN [MI]
- Midecamycin acetate (JP17)
- 3T48CPS7U2
- MOM
- DTXSID60905087
- MIDECAMYCIN ACETATE [WHO-DD]
- Leucomycin V, 3(sup B),9-diacetate 3,4(sup B)-dipropanoate
- Miocamycine
- Myocamicin
- 9,3''-Diacetylmidecamycin
- maidimeisu
- Macroral
- Miocamen
- Mosil
- ((4R,5S,6S,7R,9R,10R,11E,13E,16R)-10-acetyloxy-6-((2S,3R,4R,5S,6R)-5-((2S,4R,5S,6S)-4-acetyloxy-4,6-dimethyl-5-propanoyloxyoxan-2-yl)oxy-4-(dimethylamino)-3-hydroxy-6-methyloxan-2-yl)oxy-5-methoxy-9,16-dimethyl-2-oxo-7-(2-oxoethyl)-1-oxacyclohexadeca-11,13-dien-4-yl) propanoate
- (3R,4S,5S,6R,8R,9R,10E,12E,15R)-9-Acetoxy-5-(3-O-acetyl-2,6-dideoxy-3-C-methyl-4-O-propanoyl-alpha-L-ribo-hexopyranosyl-(1->4)-3,6-dideoxy-3-dimethylamino-beta-D-glucopyranosyloxy)-6-formylmethyl-4-methoxy-8-methyl-3-propioyloxyhexadeca-10,12-dien-15-olide
- (3R,4S,5S,6R,8R,9R,10E,12E,15R)-9-Acetoxy-5-[3-O-acetyl-2,6-dideoxy-3-C-methyl-4-O-propanoyl-alpha-L-ribo-hexopyranosyl-(1->4)-3,6-dideoxy-3-dimethylamino-beta-D-glucopyranosyloxy]-6-formylmethyl-4-methoxy-8-methyl-3-propioyloxyhexadeca-10,12-dien-15-olide
- 9,3'' Diacetylmidecamycin
- SCHEMBL139293
- 9,3'' Di O Acetylmidecamycin
- CHEMBL1091024
- GQNZGCARKRHPOH-RQIKCTSVSA-N
- DTXCID801334189
- HY-B1921
- 1532-RB
- 1ST164052
- CS-0013976
- NS00011667
- D01636
- Q3858659
- W-105540
- BRD-K30764557-001-01-5
- Midecamycin acetate, Antibiotic for Culture Media Use Only
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
898.0 g/mol
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
XLogP3-AA
Property Value
3.8
Reference
Computed by XLogP3 3.0 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Donor Count
Property Value
1
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Acceptor Count
Property Value
18
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Rotatable Bond Count
Property Value
18
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Exact Mass
Property Value
897.47219980 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Monoisotopic Mass
Property Value
897.47219980 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Topological Polar Surface Area
Property Value
218 Ų
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Heavy Atom Count
Property Value
63
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
1590
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
16
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
2
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
1
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2021.10.14)
Pharmaceuticals -> Antibiotics
S6 | ITNANTIBIOTIC | Antibiotic List from the ITN MSCA ANSWER | DOI:10.5281/zenodo.2621956
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Same Connectivity Count
Same Parent, Connectivity Count
Same Parent, Exact Count
Mixtures, Components, and Neutralized Forms Count
Similar Compounds (2D)
Similar Conformers (3D)
Taxonomy Count
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
GHS Hazard Statements
Not Classified
Reported as not meeting GHS hazard criteria by 1 of 1 companies. For more detailed information, please visit ECHA C&L website.
Not Classified
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/
- ChemIDplusMidecamycin acetate [JAN]https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0055881077ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- DrugBankLICENSECreative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode)https://www.drugbank.ca/legal/terms_of_useMiocamycinhttps://www.drugbank.ca/drugs/DB13287
- EPA DSSToxMidecamycin acetatehttps://comptox.epa.gov/dashboard/DTXSID60905087CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-noticeLeucomycin V, 3B,9-diacetate 3,4B-dipropanoatehttps://echa.europa.eu/substance-information/-/substanceinfo/100.054.418Leucomycin V, 3B,9-diacetate 3,4B-dipropanoate (EC: 259-879-6)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/53547
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingMIDECAMYCIN ACETATEhttps://gsrs.ncats.nih.gov/ginas/app/beta/substances/3T48CPS7U2
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- Open TargetsLICENSEDatasets generated by the Open Targets Platform are freely available for download.https://platform-docs.opentargets.org/licence
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.htmlAnatomical Therapeutic Chemical (ATC) classificationhttp://www.genome.jp/kegg-bin/get_htext?br08303.kegAntiinfectiveshttp://www.genome.jp/kegg-bin/get_htext?br08307.kegDrugs listed in the Japanese Pharmacopoeiahttp://www.genome.jp/kegg-bin/get_htext?br08311.keg
- Metabolomics Workbench
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/MiocamycinNORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- Therapeutic Target Database (TTD)
- WHO Anatomical Therapeutic Chemical (ATC) ClassificationLICENSEUse of all or parts of the material requires reference to the WHO Collaborating Centre for Drug Statistics Methodology. Copying and distribution for commercial purposes is not allowed. Changing or manipulating the material is not allowed.https://www.whocc.no/copyright_disclaimer/
- Wikidatamidecamycin acetatehttps://www.wikidata.org/wiki/Q3858659
- WikipediaLaquinimodhttps://en.wikipedia.org/wiki/LaquinimodMiocamycinhttps://en.wikipedia.org/wiki/Miocamycin
- PubChem
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlmidecamycinhttps://www.ncbi.nlm.nih.gov/mesh/67026483Anti-Bacterial Agentshttps://www.ncbi.nlm.nih.gov/mesh/68000900
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
CONTENTS