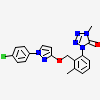
Metyltetraprole
PubChem CID
89881183
Molecular Formula
Synonyms
- metyltetraprole
- 1472649-01-6
- Metyltetraprole [ISO]
- Pavecto
- 44WE6KNK7M
Molecular Weight
396.8 g/mol
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Dates
- Create:2015-02-13
- Modify:2025-01-18
Description
Metyltetraprole is a member of the class of tetrazoles that is 1-methyl-4-phenyltetrazole in which the phenyl group has been substituted at positions 2 and 3 by [1-(p-chlorophenyl)-1H-pyrazol-3-yl]oxy}methyl and methyl groups, respectively. A quinone outside inhibitor, it is a fungicide that can be used to control a broad range of diseases, including Septoria leaf blotch in wheat. It has a role as an antifungal agrochemical and a quinone outside inhibitor. It is a member of tetrazoles, a pyrazole pesticide and a member of monochlorobenzenes.
Chemical Structure Depiction
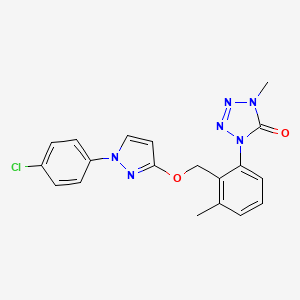
1-[2-[[1-(4-chlorophenyl)pyrazol-3-yl]oxymethyl]-3-methylphenyl]-4-methyltetrazol-5-one
Computed by Lexichem TK 2.7.0 (PubChem release 2021.10.14)
InChI=1S/C19H17ClN6O2/c1-13-4-3-5-17(26-19(27)24(2)22-23-26)16(13)12-28-18-10-11-25(21-18)15-8-6-14(20)7-9-15/h3-11H,12H2,1-2H3
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
XUQQRGKFXLAPNV-UHFFFAOYSA-N
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
CC1=C(C(=CC=C1)N2C(=O)N(N=N2)C)COC3=NN(C=C3)C4=CC=C(C=C4)Cl
Computed by OEChem 2.3.0 (PubChem release 2024.12.12)
C19H17ClN6O2
Computed by PubChem 2.2 (PubChem release 2021.10.14)
- metyltetraprole
- 1472649-01-6
- Metyltetraprole [ISO]
- Pavecto
- 44WE6KNK7M
- CHEBI:141152
- 1-(2-((1-(4-Chlorophenyl)pyrazol-3-yl)oxymethyl)-3-methylphenyl)-4-methyltetrazol-5-one
- A1D6P
- 1-(2-(((1-(4-Chlorophenyl)-1H-pyrazol-3-yl)oxy)methyl)-3-methylphenyl)-1,4-dihydro-4-methyl-5H-tetrazol-5-one
- 1-(2-((1-(4-Chlorophenyl)-1H-pyrazol-3-yl)oxymethyl)-3-methylphenyl)-4-methyl-1,4-dihydrotetrazol-5-one
- 5H-Tetrazol-5-one, 1-(2-(((1-(4-chlorophenyl)-1H-pyrazol-3-yl)oxy)methyl)-3-methylphenyl)-1,4-dihydro-4-methyl-
- 1-[2-[[1-(4-chlorophenyl)pyrazol-3-yl]oxymethyl]-3-methylphenyl]-4-methyltetrazol-5-one
- 1-(2-{[1-(4-chlorophenyl)-1H-pyrazol-3-yl]oxymethyl}-3-methylphenyl)-1,4-dihydro-4-methyl-5H-tetrazol-5-one
- 1-[2-({[1-(4-chlorophenyl)-1H-pyrazol-3-yl]oxy}methyl)-3-methylphenyl]-4-methyl-1,4-dihydro-5H-tetrazol-5-one
- 1-[2-[[[1-(4-chlorophenyl)-1H-pyrazol-3-yl]oxy]methyl]-3-methylphenyl]-1,4-dihydro-4-methyl-5H-tetrazol-5-one
- 1-(2-(((1-(4-chlorophenyl)-1H-pyrazol-3-yl)oxy)methyl)-3-methylphenyl)-4-methyl-1,4-dihydro-5H-tetrazol-5-one
- 1-(2-((1-(4-chlorophenyl)-1H-pyrazol-3-yl)oxymethyl)-3-methylphenyl)-1,4-dihydro-4-methyl-5H-tetrazol-5-one
- 1-[2-[[[1-(4-Chlorophenyl)-1H-pyrazol-3-yl]oxy]methyl]-3-methylphenyl]-1,4-dihydro-4-methyl-5H-tetrazol-5-one; 1-(2-[[1-(4-Chlorophenyl)-1H-pyrazol-3-yl]oxymethyl]-3-methylphenyl)-4-methyl-1,4-dihydrotetrazol-5-one; 1-[2-[[1-(4-Chlorophenyl)pyrazol-3-yl]oxymethyl]-3-methylphenyl]-4-methyltetrazol-5-one
- UNII-44WE6KNK7M
- SCHEMBL15359508
- DTXSID601108889
- DA-65425
- HY-146145
- CS-0527274
- Metyltetraprole 100 microg/mL in Acetonitrile
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
396.8 g/mol
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
XLogP3-AA
Property Value
4.6
Reference
Computed by XLogP3 3.0 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Donor Count
Property Value
0
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Acceptor Count
Property Value
5
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Rotatable Bond Count
Property Value
5
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Exact Mass
Property Value
396.1101515 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Monoisotopic Mass
Property Value
396.1101515 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Topological Polar Surface Area
Property Value
75.3 Ų
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Heavy Atom Count
Property Value
28
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
585
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
1
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2021.10.14)
Herbicides
Active substance -> EU Pesticides database: Pending
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Similar Compounds (2D)
Similar Conformers (3D)
Same Count
Protein Structures Count
Pesticide active substances -> Herbicides
Active Substance
metyltetraprole
Status
Pending [Reg. (EC) No 1107/2009]
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=XUQQRGKFXLAPNV-UHFFFAOYSA-N
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/1-[2-[[[1-(4-Chlorophenyl)-1H-pyrazol-3-yl]oxy]methyl]-3-methylphenyl]-1,4-dihydro-4-methyl-5H-tetrazol-5-onehttps://commonchemistry.cas.org/detail?cas_rn=1472649-01-6
- ChemIDplusChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA DSSToxMetyltetraprolehttps://comptox.epa.gov/dashboard/DTXSID601108889CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- ChEBIMetyltetraprolehttps://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:141152
- EU Pesticides Database
- RCSB Protein Data Bank (RCSB PDB)LICENSEData files contained in the PDB archive (ftp://ftp.wwpdb.org) are free of all copyright restrictions and made fully and freely available for both non-commercial and commercial use. Users of the data should attribute the original authors of that structural data.https://www.rcsb.org/pages/policies
- Springer Nature
- Thieme ChemistryLICENSEThe Thieme Chemistry contribution within PubChem is provided under a CC-BY-NC-ND 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc-nd/4.0/
- Wikidatametyltetraprolehttps://www.wikidata.org/wiki/Q106041264
- WikipediaIcenticaftorhttps://en.wikipedia.org/wiki/IcenticaftorMetyltetraprolehttps://en.wikipedia.org/wiki/Metyltetraprole
- PubChem
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 394071453https://pubchem.ncbi.nlm.nih.gov/substance/394071453
CONTENTS