Methyl phenyldithiocarbamate
PubChem CID
3032344
Molecular Formula
Synonyms
- Methyl phenyldithiocarbamate
- Methyl dithiocarbanilate
- Methyl N-phenyldithiocarbamate
- 701-73-5
- Carbamodithioic acid, phenyl-, methyl ester
Molecular Weight
183.3 g/mol
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Dates
- Create:2005-03-26
- Modify:2025-01-11
Chemical Structure Depiction
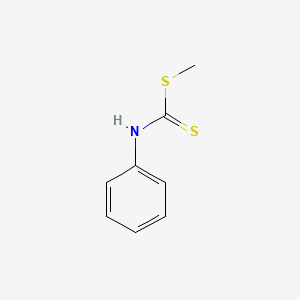
methyl N-phenylcarbamodithioate
Computed by Lexichem TK 2.7.0 (PubChem release 2021.10.14)
InChI=1S/C8H9NS2/c1-11-8(10)9-7-5-3-2-4-6-7/h2-6H,1H3,(H,9,10)
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
MOOSRNWDPWTOBF-UHFFFAOYSA-N
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
CSC(=S)NC1=CC=CC=C1
Computed by OEChem 2.3.0 (PubChem release 2024.12.12)
C8H9NS2
Computed by PubChem 2.2 (PubChem release 2021.10.14)
- Methyl phenyldithiocarbamate
- Methyl dithiocarbanilate
- Methyl N-phenyldithiocarbamate
- 701-73-5
- Carbamodithioic acid, phenyl-, methyl ester
- ENT 31472
- CARBANILIC ACID, DITHIO-, METHYL ESTER
- YOZ2OB6RH0
- NSC-57602
- Phenyldithiocarbamic acid methyl ester
- Carbamodithioic acid, N-phenyl-, methyl ester
- Methyl phenylcarbamodithioate
- NSC 57602
- BRN 2803060
- AI3-31472
- ENT-31472
- methylphenyldithiocarbamate
- UNII-YOZ2OB6RH0
- WLN: SUYS1&MR
- Carbamodithioic acid, phenyl-, methyl ester (9CI)
- SCHEMBL1286315
- SCHEMBL14285667
- DTXSID80220380
- NSC57602
- AKOS000269301
- N-PHENYL-S-METHYLDITHIOCARBAMATE
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
183.3 g/mol
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
XLogP3
Property Value
3
Reference
Computed by XLogP3 3.0 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Donor Count
Property Value
1
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Acceptor Count
Property Value
2
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Rotatable Bond Count
Property Value
2
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Exact Mass
Property Value
183.01764164 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Monoisotopic Mass
Property Value
183.01764164 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Topological Polar Surface Area
Property Value
69.4 Ų
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Heavy Atom Count
Property Value
11
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
130
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
1
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2021.10.14)
MoNA ID
MS Category
Experimental
MS Type
GC-MS
MS Level
MS1
Instrument
Unknown
Instrument Type
EI-B
Ionization Mode
positive
Top 5 Peaks
77 99.99
135 99.59
51 53.67
136 48.17
47 29.70
License
CC BY-NC-SA
MoNA ID
MS Category
Experimental
MS Type
GC-MS
MS Level
MS1
Instrument
HITACHI M-60
Instrument Type
EI-B
Ionization Mode
positive
Top 5 Peaks
77 99.99
135 99.59
51 53.67
136 48.17
47 29.70
License
CC BY-NC-SA
Accession ID
Authors
MASS SPECTROSCOPY SOC. OF JAPAN (MSSJ)
Instrument
Unknown
Instrument Type
EI-B
MS Level
MS
Ionization Mode
POSITIVE
Top 5 Peaks
77 999
135 996
51 537
136 482
47 297
License
CC BY-NC-SA
Accession ID
Authors
UBE SCIENTIFIC ANALYSIS LABORATORY
Instrument
HITACHI M-60
Instrument Type
EI-B
MS Level
MS
Ionization Mode
POSITIVE
Ionization
ENERGY 70 eV
Top 5 Peaks
77 999
135 996
51 537
136 482
47 297
License
CC BY-NC-SA
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Same Parent, Exact Count
Mixtures, Components, and Neutralized Forms Count
Similar Compounds (2D)
Similar Conformers (3D)
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=MOOSRNWDPWTOBF-UHFFFAOYSA-N
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/Methyl N-phenylcarbamodithioatehttps://commonchemistry.cas.org/detail?cas_rn=701-73-5
- ChemIDplusMethyl dithiocarbanilatehttps://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000701735ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- DTP/NCILICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuse
- EPA DSSToxCarbamodithioic acid, phenyl-, methyl ester (9CI)https://comptox.epa.gov/dashboard/DTXSID80220380CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingMETHYL DITHIOCARBANILATEhttps://gsrs.ncats.nih.gov/ginas/app/beta/substances/YOZ2OB6RH0
- Japan Chemical Substance Dictionary (Nikkaji)
- MassBank EuropeMETHYL N-PHENYLDITHIOCARBAMATEhttps://massbank.eu/MassBank/Result.jsp?inchikey=MOOSRNWDPWTOBF-UHFFFAOYSA-N
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- NIST Mass Spectrometry Data CenterLICENSEData covered by the Standard Reference Data Act of 1968 as amended.https://www.nist.gov/srd/public-lawCarbamodithioic acid, phenyl-, methyl esterhttp://www.nist.gov/srd/nist1a.cfm
- SpectraBaseCarbamodithioic acid, phenyl-, methyl esterhttps://spectrabase.com/spectrum/LzAIFC1hhCMethyl n-phenyldithiocarbamatehttps://spectrabase.com/spectrum/4kwbsIGBRg
- Springer Nature
- Thieme ChemistryLICENSEThe Thieme Chemistry contribution within PubChem is provided under a CC-BY-NC-ND 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc-nd/4.0/
- WikidataCarbamodithioic acid, phenyl-, methyl ester (9CI)https://www.wikidata.org/wiki/Q83097583
- Wiley
- PubChem
- EPA Substance Registry ServicesEPA SRS List Classificationhttps://sor.epa.gov/sor_internet/registry/substreg/LandingPage.do
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 389022812https://pubchem.ncbi.nlm.nih.gov/substance/389022812
CONTENTS