Malate
PubChem CID
160434
Molecular Formula
Synonyms
- malate
- 2-hydroxybutanedioate
- 149-61-1
- malate dianion
- malate(2-)
Molecular Weight
132.07 g/mol
Computed by PubChem 2.2 (PubChem release 2024.11.20)
Parent Compound
Dates
- Create:2004-09-16
- Modify:2025-01-18
Description
Malate(2-) is a C4-dicarboxylate resulting from deprotonation of both carboxy groups of malic acid. It has a role as a fundamental metabolite. It is a C4-dicarboxylate and a malate. It is functionally related to a succinate(2-). It is a conjugate base of a malic acid.
Chemical Structure Depiction
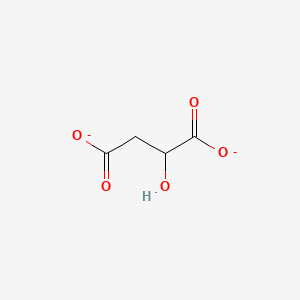
2-hydroxybutanedioate
Computed by Lexichem TK 2.7.0 (PubChem release 2024.11.20)
InChI=1S/C4H6O5/c5-2(4(8)9)1-3(6)7/h2,5H,1H2,(H,6,7)(H,8,9)/p-2
Computed by InChI 1.07.0 (PubChem release 2024.11.20)
BJEPYKJPYRNKOW-UHFFFAOYSA-L
Computed by InChI 1.07.0 (PubChem release 2024.11.20)
C(C(C(=O)[O-])O)C(=O)[O-]
Computed by OEChem 2.3.0 (PubChem release 2024.12.12)
C4H4O5-2
Computed by PubChem 2.2 (PubChem release 2024.11.20)
6204-95-1
- calcium (hydroxy-1-malate) hexahydrate
- malate
- malic acid
- malic acid, (R)-isomer
- malic acid, calcium salt, (1:1), (S)-isomer
- malic acid, disodium salt
- malic acid, disodium salt, (R)-isomer
- malic acid, disodium salt, (S)-isomer
- malic acid, magnesium salt (2:1)
- malic acid, monopotassium salt, (+-)-isomer
- malic acid, potassium salt, (R)-isomer
- malic acid, sodium salt, (+-)-isomer
- malate
- 2-hydroxybutanedioate
- 149-61-1
- malate dianion
- malate(2-)
- ANION STANDARD - MALATE
- Butanedioic acid, 2-hydroxy-, ion(2-)
- DL-Apple Acid
- Hydroxybutanedioate
- CHEMBL182856
- DL Malate
- Malate ion(2-)
- Hydroxysuccinate
- malate anion
- DL-Malate
- 3-hydroxysuccinate
- 2-Hydroxysuccinate
- Sodium; L-Malate
- (RS)-malic acid
- (RS)-malate
- Butanedioic acid, hydroxy-, ion(2)-
- CHEBI:15595
- DTXSID00933521
- BJEPYKJPYRNKOW-UHFFFAOYSA-L
- hydroxybutanedioic acid, ion(2-)
- BDBM50159798
- calcium (hydroxy-1-malate) hexahydrate
- Q27098132
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
132.07 g/mol
Reference
Computed by PubChem 2.2 (PubChem release 2024.11.20)
Property Name
XLogP3-AA
Property Value
0
Reference
Computed by XLogP3 3.0 (PubChem release 2024.11.20)
Property Name
Hydrogen Bond Donor Count
Property Value
1
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2024.11.20)
Property Name
Hydrogen Bond Acceptor Count
Property Value
5
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2024.11.20)
Property Name
Rotatable Bond Count
Property Value
1
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2024.11.20)
Property Name
Exact Mass
Property Value
132.00587322 Da
Reference
Computed by PubChem 2.2 (PubChem release 2024.11.20)
Property Name
Monoisotopic Mass
Property Value
132.00587322 Da
Reference
Computed by PubChem 2.2 (PubChem release 2024.11.20)
Property Name
Topological Polar Surface Area
Property Value
100 Ų
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2024.11.20)
Property Name
Heavy Atom Count
Property Value
9
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
-2
Reference
Computed by PubChem
Property Name
Complexity
Property Value
118
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2024.11.20)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
1
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
1
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2021.10.14)
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Same Connectivity Count
Same Stereo Count
Same Isotope Count
Same Parent, Connectivity Count
Same Parent, Stereo Count
Same Parent, Isotope Count
Same Parent, Exact Count
Mixtures, Components, and Neutralized Forms Count
Similar Compounds (2D)
Similar Conformers (3D)
Same Count
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=BJEPYKJPYRNKOW-UHFFFAOYSA-L
- BindingDBLICENSEAll data curated by BindingDB staff are provided under the Creative Commons Attribution 3.0 License (https://creativecommons.org/licenses/by/3.0/us/).https://www.bindingdb.org/rwd/bind/info.jsp
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/
- ChemIDplusButanedioic acid, hydroxy-, ion(2)-https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000149611ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA DSSTox2-Hydroxybutanedioatehttps://comptox.epa.gov/dashboard/DTXSID00933521CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- ChEBI
- Japan Chemical Substance Dictionary (Nikkaji)
- KNApSAcK Species-Metabolite Database
- Natural Product Activity and Species Source (NPASS)
- Nature Chemistry
- Rhea - Annotated Reactions DatabaseLICENSERhea has chosen to apply the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0/). This means that you are free to copy, distribute, display and make commercial use of the database in all legislations, provided you credit (cite) Rhea.https://www.rhea-db.org/help/license-disclaimer
- Wikidatamalate(2-)https://www.wikidata.org/wiki/Q27098132
- PubChem
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.html
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 403266804https://pubchem.ncbi.nlm.nih.gov/substance/403266804
CONTENTS

 CID 525 (Malic Acid)
CID 525 (Malic Acid)