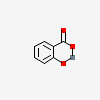
Mercuric salicylate
- Mercury salicylate
- MERCURIC SALICYLATE
- Mercurous salicylate
- EINECS 227-760-8
- UNII-W49EC1DTLR
- Create:2007-08-23
- Modify:2025-01-11
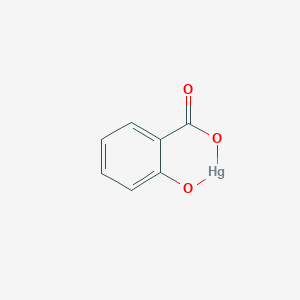
- Mercury salicylate
- MERCURIC SALICYLATE
- Mercurous salicylate
- EINECS 227-760-8
- UNII-W49EC1DTLR
- Mercuric salicylate [NF]
- UN1644
- W49EC1DTLR
- Mercury, (2-hydroxybenzoateo(2-)-O1,O2)-
- 2,4-dioxa-3-mercurabicyclo[4.4.0]deca-1(10),6,8-trien-5-one
- Mercury, (2-(hydroxy-kappaO)benzoato(2-)-kappaO)-
- MERCURIC SALICYLATE, (SOLID)
- MERCURIC SALICYLATE, [SOLID]
- Mercury subsalicylate
- MERCURY, (2-HYDROXYBENZOATO(2-)-O1,O2)-
- MERCURY, [2-HYDROXYBENZOATO(2-)-O1,O2]-
- Mercury, (salicylato(2-))-
- (Salicylato(2-)-O1,O2)mercury
- Mercury, (salicylato(2))
- Salicylic acid, (hydroxymercuri)-, cyclic anhydride
- (Salicylato(2)O1,O2)mercury
- Mercury, (2hydroxybenzoateo(2)O1,O2)
- Mercury salicylate [UN1644] [Poison]
- NS00122293
- Mercury, (2hydroxybenzoateo(2)O1,O2) (9CI)
- Salicylic acid, (hydroxymercuri), cyclic anhydride
- Mercury, (2-hydroxybenzoateo(2-)-O1,O2)-(9CI)
- Q27292286
- Salicylic acid, (hydroxymercuri), cyclic anhydride (6CI)
Excerpt from ERG Guide 151 [Substances - Toxic (Non-Combustible)]:
Highly toxic, may be fatal if inhaled, ingested or absorbed through skin. Avoid any skin contact. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause environmental contamination. (ERG, 2024)
· Highly toxic, may be fatal if inhaled, ingested or absorbed through skin.
· Avoid any skin contact.
· Fire may produce irritating, corrosive and/or toxic gases.
· Runoff from fire control or dilution water may be corrosive and/or toxic and cause environmental contamination.
Excerpt from ERG Guide 151 [Substances - Toxic (Non-Combustible)]:
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Containers may explode when heated. Runoff may pollute waterways. (ERG, 2024)
· Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes.
· Containers may explode when heated.
· Runoff may pollute waterways.
Excerpt from ERG Guide 151 [Substances - Toxic (Non-Combustible)]:
Refer to the "General First Aid" section. (ERG, 2024)
General First Aid:
· Call 911 or emergency medical service.
· Ensure that medical personnel are aware of the material(s) involved, take precautions to protect themselves and avoid contamination.
· Move victim to fresh air if it can be done safely.
· Administer oxygen if breathing is difficult.
· If victim is not breathing:
-- DO NOT perform mouth-to-mouth resuscitation; the victim may have ingestedor inhaled the substance.
-- If equipped and pulse detected, wash face and mouth, then give artificial respiration using a proper respiratory medical device (bag-valve mask, pocket mask equipped with a one-way valve or other device).
-- If no pulse detected or no respiratory medical device available, provide continuouscompressions. Conduct a pulse check every two minutes or monitor for any signs of spontaneous respirations.
· Remove and isolate contaminated clothing and shoes.
· For minor skin contact, avoid spreading material on unaffected skin.
· In case of contact with substance, remove immediately by flushing skin or eyes with running water for at least 20 minutes.
· For severe burns, immediate medical attention is required.
· Effects of exposure (inhalation, ingestion, or skin contact) to substance may be delayed.
· Keep victim calm and warm.
· Keep victim under observation.
· For further assistance, contact your local Poison Control Center.
· Note: Basic Life Support (BLS) and Advanced Life Support (ALS) should be done by trained professionals.
In Canada, an Emergency Response Assistance Plan (ERAP) may be required for this product. Please consult the shipping paper and/or the "ERAP" section.
Excerpt from ERG Guide 151 [Substances - Toxic (Non-Combustible)]:
SMALL FIRE: Dry chemical, CO2 or water spray.
LARGE FIRE: Water spray, fog or regular foam. If it can be done safely, move undamaged containers away from the area around the fire. Dike runoff from fire control for later disposal. Avoid aiming straight or solid streams directly onto the product.
FIRE INVOLVING TANKS, RAIL TANK CARS OR HIGHWAY TANKS: Fight fire from maximum distance or use unmanned master stream devices or monitor nozzles. Do not get water inside containers. Cool containers with flooding quantities of water until well after fire is out. Withdraw immediately in case of rising sound from venting safety devices or discoloration of tank. ALWAYS stay away from tanks in direct contact with flames. For massive fire, use unmanned master stream devices or monitor nozzles; if this is impossible, withdraw from area and let fire burn. (ERG, 2024)
· CALL 911. Then call emergency response telephone number on shipping paper. If shipping paper not available or no answer, refer to appropriate telephone number listed on the inside back cover.
· Keep unauthorized personnel away.
· Stay upwind, uphill and/or upstream.
· Do not touch damaged containers or spilled material unless wearing appropriate protective clothing.
· Stop leak if you can do it without risk.
· Prevent entry into waterways, sewers, basements or confined areas.
· Cover with plastic sheet to prevent spreading.
· Absorb or cover with dry earth, sand or other non-combustible material and transfer to containers.
· DO NOT GET WATER INSIDE CONTAINERS.
· For solids, prevent dust cloud and avoid inhalation of dust.
Excerpt from ERG Guide 151 [Substances - Toxic (Non-Combustible)]:
IMMEDIATE PRECAUTIONARY MEASURE: Isolate spill or leak area in all directions for at least 50 meters (150 feet) for liquids and at least 25 meters (75 feet) for solids.
SPILL: Increase the immediate precautionary measure distance, in the downwind direction, as necessary.
FIRE: If tank, rail tank car or highway tank is involved in a fire, ISOLATE for 800 meters (1/2 mile) in all directions; also, consider initial evacuation for 800 meters (1/2 mile) in all directions. (ERG, 2024)
Immediate precautionary measure
· Isolate spill or leak area in all directions for at least 50 meters (150 feet) for liquids and at least 25 meters (75 feet) for solids.
Spill
· For highlighted materials: see Table 1 - Initial Isolation and Protective Action Distances.
· For non-highlighted materials: increase the immediate precautionary measure distance, in the downwind direction, as necessary.
Fire
· If tank, rail tank car or highway tank is involved in a fire, ISOLATE for 800 meters (1/2 mile) in all directions; also, consider initial evacuation for 800 meters (1/2 mile) in all directions.
Excerpt from ERG Guide 151 [Substances - Toxic (Non-Combustible)]:
Do not touch damaged containers or spilled material unless wearing appropriate protective clothing. Stop leak if you can do it without risk. Prevent entry into waterways, sewers, basements or confined areas. Cover with plastic sheet to prevent spreading. Absorb or cover with dry earth, sand or other non-combustible material and transfer to containers. DO NOT GET WATER INSIDE CONTAINERS. For solids, prevent dust cloud and avoid inhalation of dust. (ERG, 2024)
· Wear positive pressure self-contained breathing apparatus (SCBA).
· Wear chemical protective clothing that is specifically recommended by the manufacturer when there is NO RISK OF FIRE.
· Structural firefighters' protective clothing provides thermal protection but only limited chemical protection.
Small Fire
· Dry chemical, CO2 or water spray.
Large Fire
· Water spray, fog or regular foam.
· If it can be done safely, move undamaged containers away from the area around the fire.
· Dike runoff from fire control for later disposal.
· Avoid aiming straight or solid streams directly onto the product.
Fire Involving Tanks, Rail Tank Cars or Highway Tanks
· Fight fire from maximum distance or use unmanned master stream devices or monitor nozzles.
· Do not get water inside containers.
· Cool containers with flooding quantities of water until well after fire is out.
· Withdraw immediately in case of rising sound from venting safety devices or discoloration of tank.
· ALWAYS stay away from tanks in direct contact with flames.
· For massive fire, use unmanned master stream devices or monitor nozzles; if this is impossible, withdraw from area and let fire burn.
Excerpt from ERG Guide 151 [Substances - Toxic (Non-Combustible)]:
Wear positive pressure self-contained breathing apparatus (SCBA). Wear chemical protective clothing that is specifically recommended by the manufacturer when there is NO RISK OF FIRE. Structural firefighters' protective clothing provides thermal protection but only limited chemical protection. (ERG, 2024)
Phenols and Cresols
Salts, Basic
Neurotoxin - Sensorimotor
Nephrotoxin - The chemical is potentially toxic to the kidneys in the occupational setting.
- CAMEO ChemicalsLICENSECAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data.https://cameochemicals.noaa.gov/help/reference/terms_and_conditions.htm?d_f=falseMERCURY SALICYLATEhttps://cameochemicals.noaa.gov/chemical/1045CAMEO Chemical Reactivity Classificationhttps://cameochemicals.noaa.gov/browse/react
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/Mercury salicylatehttps://commonchemistry.cas.org/detail?cas_rn=5970-32-1
- ChemIDplusMercuric salicylate [NF]https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0005970321ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- Emergency Response Guidebook (ERG)Mercury salicylatehttps://pubchem.ncbi.nlm.nih.gov/erg/
- Haz-Map, Information on Hazardous Chemicals and Occupational DiseasesLICENSECopyright (c) 2022 Haz-Map(R). All rights reserved. Unless otherwise indicated, all materials from Haz-Map are copyrighted by Haz-Map(R). No part of these materials, either text or image may be used for any purpose other than for personal use. Therefore, reproduction, modification, storage in a retrieval system or retransmission, in any form or by any means, electronic, mechanical or otherwise, for reasons other than personal use, is strictly prohibited without prior written permission.https://haz-map.com/AboutMercuric salicylatehttps://haz-map.com/Agents/2566
- Wikidatamercuric salicylatehttps://www.wikidata.org/wiki/Q27292286
- PubChem
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/NORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- NCBI