L-Palmitoylcarnitine
- L-Palmitoylcarnitine
- Palmitoyl-L-carnitine
- 2364-67-2
- O-palmitoyl-L-carnitine
- Hexadecanoyl-L-carnitine
- Create:2006-11-22
- Modify:2025-01-18
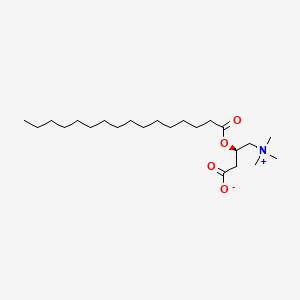
- Hexadecanoylcarnitine
- Palmitoylcarnitine
- Palmitylcarnitine
- L-Palmitoylcarnitine
- Palmitoyl-L-carnitine
- 2364-67-2
- O-palmitoyl-L-carnitine
- Hexadecanoyl-L-carnitine
- Palmityl-L-carnitine
- L-Carnitine palmitoyl ester
- PALMITOYLCARNITINE
- palmitoyl carnitine
- O-hexadecanoyl-R-carnitine
- Hexadecenoyl carnitine
- CHEBI:17490
- L(-)-Palmitylcarnitine
- Palmitoyl-(-)-carnitine
- Palmitoyl carnitine, (-)-
- (3R)-3-hexadecanoyloxy-4-(trimethylazaniumyl)butanoate
- CHEMBL3392050
- 1G38O5K038
- Ammonium, (3-carboxy-2-hydroxypropyl)trimethyl-, hydroxide, inner salt, palmitate, L-
- Palmitylcarnitine
- (3R)-3-(hexadecanoyloxy)-4-(trimethylazaniumyl)butanoate
- D-Palmitylcarnitine
- Palmitoyl d-carnitine
- palmitoyl L-carnitine
- Palmitoyl-L-(-)-carnitin
- UNII-1G38O5K038
- Palmitoyl-L-(-)-carnitin [German]
- L-Palmitoyl carnitine
- L-palmitoyl-L-carnitine
- (-)-palmitoyl carnitine
- O-hexadecanoyl-L-carnitine
- O-hexadecanoyl-(R)-carnitine
- SCHEMBL116634
- XOMRRQXKHMYMOC-OAQYLSRUSA-N
- DTXSID101019091
- BDBM50537024
- CAR(16:0)
- LMFA07070004
- AKOS037645114
- AS-57239
- PD101999
- XP165791
- Palmitoyl-L-carnitine, >=97.0% (TLC)
- HY-113147
- CS-0062278
- C16 Carnitine, palmitoyl L-carnitine, powder
- C02990
- G91203
- (3R)-3-palmitoyloxy-4-(trimethylammonio)butanoate
- (3R)-3-hexadecanoyloxy-4-(trimethylammonio)butanoate
- (3S)-3-palmitoyloxy-4-(trimethylammonio)butanoic acid
- Q27102424
- (3S)-3-hexadecanoyloxy-4-(trimethylammonio)butanoic acid
- 3-carboxy-N,N,N-trimethyl-2-[(1-oxohexadecyl)oxy]-1-Propanaminium
- 1-PROPANAMINIUM, 3-CARBOXY-N,N,N-TRIMETHYL-2-((1-OXOHEXADECYL)OXY)-, INNER SALT, (2R)-
- 1-PROPANAMINIUM, 3-CARBOXY-N,N,N-TRIMETHYL-2-((1-OXOHEXADECYL)OXY)-, INNER SALT, (R)-
- PALMITIC ACID, ESTER WITH (3-CARBOXY-2-HYDROXYPROPYL)TRIMETHYLAMMONIUM HYDROXIDE INNER SALT, L-
214 Ų [M+H]+ [CCS Type: DT; Method: single field calibrated with Agilent tune mix (Agilent)]
204.49 Ų [M-H]- [CCS Type: DT; Method: single field calibrated with Agilent tune mix (Agilent)]
400.3427 999
85.0288 675
60.0817 85
341.268 77
239.2359 24
400.3423 999
402.349 32
85.0277 6
341.268 2
401.345398 100
341.26889 89.19
239.236969 22.07
342.2724 7.42
257.247559 2.76
400.341309 100
401.344635 27.97
801.681946 3.28
341.268341 2.32
400.445862 1.59
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=XOMRRQXKHMYMOC-OAQYLSRUSA-N
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/Palmitoyl-(-)-carnitinehttps://commonchemistry.cas.org/detail?cas_rn=2364-67-2
- ChemIDplusPalmitoyl-L-carnitinehttps://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0002364672ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA DSSToxPalmitoyl-L-carnitinehttps://comptox.epa.gov/dashboard/DTXSID101019091CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingPALMITOYL CARNITINE, (-)-https://gsrs.ncats.nih.gov/ginas/app/beta/substances/1G38O5K038
- CCSbaseCCSbase Classificationhttps://ccsbase.net/
- ChEBIO-palmitoyl-L-carnitinehttps://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:17490
- E. coli Metabolome Database (ECMDB)
- LOTUS - the natural products occurrence databaseLICENSEThe code for LOTUS is released under the GNU General Public License v3.0.https://lotus.nprod.net/L-Palmitoylcarnitinehttps://www.wikidata.org/wiki/Q27102424LOTUS Treehttps://lotus.naturalproducts.net/
- Yeast Metabolome Database (YMDB)L-Palmitoylcarnitinehttps://www.ymdb.ca/compounds/YMDB01533Hexadecanoylcarnitinehttps://www.ymdb.ca/compounds/YMDB01534
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- Cosmetic Ingredient Review (CIR)
- EPA Chemical and Products Database (CPDat)Palmitoyl-L-carnitinehttps://comptox.epa.gov/dashboard/DTXSID101019091#exposureEPA CPDat Classificationhttps://www.epa.gov/chemical-research/chemical-and-products-database-cpdat
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/NORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- ECI Group, LCSB, University of LuxembourgO-palmitoyl-L-carnitine
- KNApSAcK Species-Metabolite DatabaseHexadecanoyl-L-carnitinehttp://www.knapsackfamily.com/knapsack_core/info.php?sname=C_ID&word=C00052312
- Natural Product Activity and Species Source (NPASS)(3R)-3-Hexadecanoyloxy-4-(Trimethylazaniumyl)Butanoatehttps://bidd.group/NPASS/compound.php?compoundID=NPC326651
- FooDBLICENSEFooDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (FooDB) and the original publication.https://foodb.ca/aboutL-Palmitoylcarnitinehttps://foodb.ca/compounds/FDB021910
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.html
- LIPID MAPSLipid Classificationhttps://www.lipidmaps.org/
- MassBank Europe
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- Metabolomics WorkbenchPalmitoylcarnitinehttps://www.metabolomicsworkbench.org/data/StructureData.php?RegNo=4484L-palmitoylcarnitinehttps://www.metabolomicsworkbench.org/data/StructureData.php?RegNo=50935
- Nature Chemical Biology
- NIST Mass Spectrometry Data CenterLICENSEData covered by the Standard Reference Data Act of 1968 as amended.https://www.nist.gov/srd/public-lawPalmitoylcarnitinehttp://www.nist.gov/srd/nist1a.cfm
- PharosLICENSEData accessed from Pharos and TCRD is publicly available from the primary sources listed above. Please respect their individual licenses regarding proper use and redistribution.https://pharos.nih.gov/aboutPalmitoyl L-carnitinehttps://pharos.nih.gov/ligands/R259LVLQ1VY1
- Rhea - Annotated Reactions DatabaseLICENSERhea has chosen to apply the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0/). This means that you are free to copy, distribute, display and make commercial use of the database in all legislations, provided you credit (cite) Rhea.https://www.rhea-db.org/help/license-disclaimer
- Springer Nature
- Wikidata(-)-palmitoyl carnitinehttps://www.wikidata.org/wiki/Q27102424
- WikipediaPalmitoylcarnitinehttps://en.wikipedia.org/wiki/Palmitoylcarnitine
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlPalmitoylcarnitinehttps://www.ncbi.nlm.nih.gov/mesh/68010172Vitamin B Complexhttps://www.ncbi.nlm.nih.gov/mesh/68014803
- PubChem
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 396169442https://pubchem.ncbi.nlm.nih.gov/substance/396169442
 CID 167760 (Tocris-0609)
CID 167760 (Tocris-0609)


