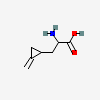
Hypoglycine A
PubChem CID
9081
Molecular Formula
Synonyms
- Hypoglycine
- Hypoglycine A
- 2-Methylenecyclopropanylalanine
- Hypoglycin A
- Cyclopropanealanine, 2-methylene-
Molecular Weight
141.17 g/mol
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Dates
- Create:2005-03-26
- Modify:2024-12-27
Description
2-amino-3-(2-methylenecyclopropyl)propanoic acid is a non-proteinogenic alpha-amino acid that is alanine in which one of the methyl hydrogens has been replaced by a 2-methylenecyclopropyl group. The phytotoxin known as hypoglycin A is a mixture of the diastereoisomers that have L configuration at the amino-bearing carbon. It is a non-proteinogenic alpha-amino acid, a member of cyclopropanes and an olefinic compound.
Hypoglycine A has been reported in Dimocarpus longan, Nephelium ramboutan-ake, and Blighia sapida with data available.
Chemical Structure Depiction

2-amino-3-(2-methylidenecyclopropyl)propanoic acid
Computed by Lexichem TK 2.7.0 (PubChem release 2021.10.14)
InChI=1S/C7H11NO2/c1-4-2-5(4)3-6(8)7(9)10/h5-6H,1-3,8H2,(H,9,10)
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
OOJZCXFXPZGUBJ-UHFFFAOYSA-N
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
C=C1CC1CC(C(=O)O)N
Computed by OEChem 2.3.0 (PubChem release 2021.10.14)
C7H11NO2
Computed by PubChem 2.2 (PubChem release 2021.10.14)
156-56-9
- hypoglycin
- hypoglycin A
- hypoglycin, (S)-isomer
- hypoglycin, carboxy-(14)C-labeled
- Hypoglycine
- Hypoglycine A
- 2-Methylenecyclopropanylalanine
- Hypoglycin A
- Cyclopropanealanine, 2-methylene-
- .beta.-(Methylenecyclopropyl)alanine
- 2-Amino-4,5-methylenehex-5-enoic acid
- beta-(Methylenecyclopropyl)alanine
- .alpha.-Aminomethylenecyclopropanepropionic acid
- NSC 303803
- 2-amino-3-(2-methylenecyclopropyl)propanoic acid
- (S)-Hypoglycine A, 85%
- .alpha.-Amino-2-methylenecyclopropanepropionic acid
- Cyclopropanepropanoic acid, .alpha.-amino-2-methylene-
- alpha-Aminomethylenecyclopropanepropionic acid
- S-Hypoglycine A
- L-.alpha.-Amino-.beta.-methylenecyclopropanepropionic acid
- .alpha.-Amino-.beta.-(2-methylenecyclopropyl)propionic acid
- alpha-Amino-2-methylenecyclopropanepropanoic acid
- alpha-Amino-2-methylenecyclopropanepropionic acid
- L-alpha-Amino-beta-methylenecyclopropanepropionic acid
- alpha-Amino-beta-(2-methylenecyclopropyl)propionic acid
- L-Hypoglycin A
- NSC-303803
- Cyclopropanealanine, L-
- SCHEMBL956162
- Cyclopropanepropionic acid, .alpha.-amino-2-methylene-, L-(+)-
- 2-MethyleneL-Cyclopropanealanine
- Cyclopropanepropanoic acid, .alpha.-amino-2-methylene-, (.alpha.S,1R)-
- WLN: L3YTJ AU1 B1YZVQ
- DTXSID50871621
- CHEBI:136270
- OOJZCXFXPZGUBJ-UHFFFAOYSA-N
- 3-(2-Methylenecyclopropyl)alanine
- 3-(2-Methylidenecyclopropyl)alanine
- Cyclopropanepropionic acid, L-(+)-
- 3-(2-Methylenecyclopropyl)alanine #
- NSC303803
- AKOS006277118
- NS00015432
- 2-amino-3-(2-methylenecyclopropyl)propionic acid
- J-009313
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
141.17 g/mol
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
XLogP3-AA
Property Value
-2.5
Reference
Computed by XLogP3 3.0 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Donor Count
Property Value
2
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Acceptor Count
Property Value
3
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Rotatable Bond Count
Property Value
3
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Exact Mass
Property Value
141.078978594 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Monoisotopic Mass
Property Value
141.078978594 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Topological Polar Surface Area
Property Value
63.3Ų
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Heavy Atom Count
Property Value
10
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
176
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
2
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
1
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2021.10.14)
Yellow solid; [Merck Index]
Solid
280 - 284 °C
Biological Agents -> Plant Toxins
NIST Number
133007
Library
Main library
Total Peaks
85
m/z Top Peak
74
m/z 2nd Highest
96
m/z 3rd Highest
46
Thumbnail
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Same Connectivity Count
Same Isotope Count
Same Parent, Connectivity Count
Same Parent, Isotope Count
Same Parent, Exact Count
Mixtures, Components, and Neutralized Forms Count
Similar Compounds (2D)
Similar Conformers (3D)
- Cytoplasm
- Extracellular
Sources/Uses
Hypoglycemia-inducing component of the akee plant (Blighia sapida); [Merck Index] A water-soluble liver toxin found in the seeds, pods, and unripe fruit of the ackee plant; Ackee is the national fruit of Jamaica, where it is associated with Jamaican vomiting sickness or Toxic Hypoglycemic Syndrome (THS); [HSDB]
Merck Index - O'Neil MJ, Heckelman PE, Dobbelaar PH, Roman KJ (eds). The Merck Index, An Encyclopedia of Chemicals, Drugs, and Biologicals, 15th Ed. Cambridge, UK: The Royal Society of Chemistry, 2013.
Most poisonings occurred before 1955 in Jamaica or the West Indies; There were fatal cases in preschool children. Severe hypoglycemia below 29 mg/dl is followed by depletion of liver glycogen. Hepatitis may develop. [HSDB] Hypoglycin A & B are hypoglycemic agents from the unripe fruit of the akee plant; Hypoglycemia and vomiting precede the CNS symptoms of seizures and coma; [Ford, p. 919]
Ford - Ford MD, Delaney KA, Ling LJ, Erickson T (eds). Clinical Toxicology. Philadelphia: W.B. Saunders, 2001., p. 919
Neurotoxin - Other CNS neurotoxin
Occupational hepatotoxin - Secondary hepatotoxins: the potential for toxic effect in the occupational setting is based on cases of poisoning by human ingestion or animal experimentation.
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=OOJZCXFXPZGUBJ-UHFFFAOYSA-N
The LOTUS Initiative for Open Natural Products Research: frozen dataset union wikidata (with metadata) | DOI:10.5281/zenodo.5794106
- ChEBI2-amino-3-(2-methylenecyclopropyl)propanoic acidhttps://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:136270
- LOTUS - the natural products occurrence databaseLICENSEThe code for LOTUS is released under the GNU General Public License v3.0.https://lotus.nprod.net/Hypoglycine Ahttps://www.wikidata.org/wiki/Q69999984LOTUS Treehttps://lotus.naturalproducts.net/
- DTP/NCILICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuse
- Human Metabolome Database (HMDB)LICENSEHMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications.http://www.hmdb.ca/citingL-Hypoglycin Ahttp://www.hmdb.ca/metabolites/HMDB0029427
- EPA DSSTox3-(2-Methylidenecyclopropyl)alaninehttps://comptox.epa.gov/dashboard/DTXSID50871621
- Haz-Map, Information on Hazardous Chemicals and Occupational DiseasesLICENSECopyright (c) 2022 Haz-Map(R). All rights reserved. Unless otherwise indicated, all materials from Haz-Map are copyrighted by Haz-Map(R). No part of these materials, either text or image may be used for any purpose other than for personal use. Therefore, reproduction, modification, storage in a retrieval system or retransmission, in any form or by any means, electronic, mechanical or otherwise, for reasons other than personal use, is strictly prohibited without prior written permission.https://haz-map.com/AboutHypoglycine Ahttps://haz-map.com/Agents/5600
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.html
- Metabolomics Workbench
- NIST Mass Spectrometry Data CenterLICENSEData covered by the Standard Reference Data Act of 1968 as amended.https://www.nist.gov/srd/public-lawHypoglycinhttp://www.nist.gov/srd/nist1a.cfm
- Springer Nature
- Wikidatahypoglycine ahttps://www.wikidata.org/wiki/Q69999984
- WikipediaHypoglycin Ahttps://en.wikipedia.org/wiki/Hypoglycin_A
- PubChem
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.html
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/NORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 389069066https://pubchem.ncbi.nlm.nih.gov/substance/389069066
CONTENTS