Go-6976
PubChem CID
3501
Molecular Formula
Synonyms
- 136194-77-9
- Go 6976
- GO6976
- Go-6976
- Goe 6976
Molecular Weight
378.4 g/mol
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Dates
- Create:2005-03-25
- Modify:2025-01-18
Description
Goe 6976 is an organic heterohexacyclic compound and an indolocarbazole. It has a role as an EC 2.7.11.13 (protein kinase C) inhibitor.
An inhibitor of calcium-dependent isoforms of protein kinase C.
Chemical Structure Depiction
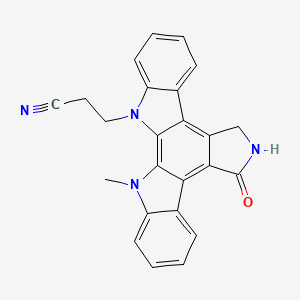
3-(23-methyl-14-oxo-3,13,23-triazahexacyclo[14.7.0.02,10.04,9.011,15.017,22]tricosa-1,4,6,8,10,15,17,19,21-nonaen-3-yl)propanenitrile
Computed by Lexichem TK 2.7.0 (PubChem release 2021.10.14)
InChI=1S/C24H18N4O/c1-27-17-9-4-2-7-14(17)20-21-16(13-26-24(21)29)19-15-8-3-5-10-18(15)28(12-6-11-25)23(19)22(20)27/h2-5,7-10H,6,12-13H2,1H3,(H,26,29)
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
VWVYILCFSYNJHF-UHFFFAOYSA-N
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
CN1C2=CC=CC=C2C3=C4C(=C5C6=CC=CC=C6N(C5=C31)CCC#N)CNC4=O
Computed by OEChem 2.3.0 (PubChem release 2024.12.12)
C24H18N4O
Computed by PubChem 2.2 (PubChem release 2021.10.14)
- Go 6976
- Go-6976
- Go6976
- Goe 6976
- 136194-77-9
- Go 6976
- GO6976
- Go-6976
- Goe 6976
- 3-(13-Methyl-5-oxo-6,7-dihydro-5H-indolo[2,3-a]pyrrolo[3,4-c]carbazol-12(13H)-yl)propanenitrile
- B9IQO7JZ16
- 12-(2-Cyanoethyl)-6,7,12,13-tetrahydro-13-methyl-5-oxo-5H-indolo[2,3-a]pyrrolo[3,4-c]carbazole
- CHEMBL302449
- CHEBI:51913
- 12H-Indolo(2,3-a)pyrrolo(3,4-c)carbazole-12-propanenitrile, 5,6,7,13-tetrahydro-13-methyl-5-oxo-
- 12H-indolo[2,3-a]pyrrolo[3,4-c]carbazole-12-propanenitrile, 5,6,7,13-tetrahydro-13-methyl-5-oxo-
- 12-(2-cyanoethyl)-6,7,12,13-tetrahydro-13-methyl-5-oxo-5H-indolo[2,3-a]pyrolo[3,4-c]carbazole
- 3-(13-methyl-5-oxo-5,6,7,13-tetrahydro-12H-indolo[2,3-a]pyrrolo[3,4-c]carbazol-12-yl)propanenitrile
- 5,6,7,13-Tetrahydro-13-Methyl-5-oxo-12H-indolo[2,3-a]pyrrolo[3,4-c]carbazole-12-propanenitrile
- UNII-B9IQO7JZ16
- Goe-6976
- C24H18N4O
- Kinome_3630
- Gouml 6976
- G ouml 6976
- CBiol_001871
- G? 6976
- BSPBio_001101
- KBioGR_000441
- KBioSS_000441
- 13-Methyl-5-oxo-5,6,7,13-tetrahydro-12H-indolo(2,3-a)pyrrolo(3,4-c)carbazole-12-propanenitrile
- BDBM3033
- GTPL5973
- SCHEMBL2175239
- BCBcMAP01_000156
- KBio2_000441
- KBio2_003009
- KBio2_005577
- KBio3_000821
- KBio3_000822
- DTXSID70159731
- Bio1_000157
- Bio1_000646
- Bio1_001135
- Bio2_000381
- Bio2_000861
- GLXC-03625
- HMS1362G03
- HMS1792G03
- HMS1990G03
- HMS3229E13
- HMS3403G03
- BCP06797
- 3-[methyl(oxo)[?]yl]propanenitrile
- EI-269
- HB0302
- MFCD00236434
- s7119
- AKOS024457007
- AT23580
- CCG-206755
- SDCCGSBI-0086678.P003
- Go6976 (PD40697)
- IDI1_002136
- NCGC00163451-01
- NCGC00163451-02
- NCGC00163451-03
- NCGC00163451-04
- AS-16804
- BG168444
- DA-63859
- GO 6976?
- HY-10183
- PD406976
- CS-0002498
- G?? 6976, >=98% (HPLC), powder
- Go 6976 - CAS 136194-77-9
- J-006821
- BRD-K59304176-001-02-5
- BRD-K59304176-001-03-3
- Q27077832
- 3-(23-methyl-14-oxo-3,13,23-triazahexacyclo[14.7.0.0^{2,10.0^{4,9.0^{11,15.0^{17,22]tricosa-1,4,6,8,10,15,17,19,21-nonaen-3-yl)propanenitrile
- 3-(23-methyl-14-oxo-3,13,23-triazahexacyclo[14.7.0.02,10.04,9.011,15.017,22]tricosa-1,4,6,8,10,15,17,19,21-nonaen-3-yl)propanenitrile
- 3-{23-methyl-14-oxo-3,13,23-triazahexacyclo[14.7.0.0(2),(1)?.0?,?.0(1)(1),(1)?.0(1)?,(2)(2)]tricosa-1(16),2(10),4(9),5,7,11(15),17(22),18,20-nonaen-3-yl}propanenitrile
- 3-{23-methyl-14-oxo-3,13,23-triazahexacyclo[14.7.0.0^{2,10}.0^{4,9}.0^{11,15}.0^{17,22}]tricosa-1(16),2(10),4(9),5,7,11(15),17,19,21-nonaen-3-yl}propanenitrile
- 3-{23-methyl-14-oxo-3,13,23-triazahexacyclo[14.7.0.0^{2,10}.0^{4,9}.0^{11,15}.0^{17,22}]tricosa-1(16),2(10),4,6,8,11(15),17,19,21-nonaen-3-yl}propanenitrile
- 5,6,7,13-Tetrahydro-13-methyl-5-oxo--12H-indolo[2,3-a]pyrrolo[3,4-c]carbazole-12-propa-nenitrile
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
378.4 g/mol
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
XLogP3-AA
Property Value
3.2
Reference
Computed by XLogP3 3.0 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Donor Count
Property Value
1
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Acceptor Count
Property Value
2
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Rotatable Bond Count
Property Value
2
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Exact Mass
Property Value
378.14806121 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Monoisotopic Mass
Property Value
378.14806121 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Topological Polar Surface Area
Property Value
62.8 Ų
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Heavy Atom Count
Property Value
29
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
730
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
1
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2021.10.14)
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Similar Compounds (2D)
Similar Conformers (3D)
Same Count
Enzyme Inhibitors
Compounds or agents that combine with an enzyme in such a manner as to prevent the normal substrate-enzyme combination and the catalytic reaction. (See all compounds classified as Enzyme Inhibitors.)
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=VWVYILCFSYNJHF-UHFFFAOYSA-N
- BindingDBLICENSEAll data curated by BindingDB staff are provided under the Creative Commons Attribution 3.0 License (https://creativecommons.org/licenses/by/3.0/us/).https://www.bindingdb.org/rwd/bind/info.jsp3-{23-methyl-14-oxo-3,13,23-triazahexacyclo[14.7.0.0^{2,10}.0^{4,9}.0^{11,15}.0^{17,22}]tricosa-1(16),2(10),4(9),5,7,11(15),17,19,21-nonaen-3-yl}propanenitrilehttps://www.bindingdb.org/rwd/bind/chemsearch/marvin/MolStructure.jsp?monomerid=3033
- Comparative Toxicogenomics Database (CTD)LICENSEIt is to be used only for research and educational purposes. Any reproduction or use for commercial purpose is prohibited without the prior express written permission of NC State University.http://ctdbase.org/about/legal.jsp
- IUPHAR/BPS Guide to PHARMACOLOGYLICENSEThe Guide to PHARMACOLOGY database is licensed under the Open Data Commons Open Database License (ODbL) https://opendatacommons.org/licenses/odbl/. Its contents are licensed under a Creative Commons Attribution-ShareAlike 4.0 International License (http://creativecommons.org/licenses/by-sa/4.0/)https://www.guidetopharmacology.org/about.jsp#licenseGuide to Pharmacology Target Classificationhttps://www.guidetopharmacology.org/targets.jsp
- Therapeutic Target Database (TTD)
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/12-(2-Cyanoethyl)-6,7,12,13-tetrahydro-13-methyl-5-oxo-5H-indolo[2,3-a]pyrrolo[3,4-c]carbazolehttps://commonchemistry.cas.org/detail?cas_rn=136194-77-9
- ChemIDplusChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- DrugBankLICENSECreative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode)https://www.drugbank.ca/legal/terms_of_use
- EPA DSSToxCompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- ChEBI
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- Japan Chemical Substance Dictionary (Nikkaji)
- Metabolomics Workbench
- Nature Chemical Biology
- Protein Data Bank in Europe (PDBe)
- RCSB Protein Data Bank (RCSB PDB)LICENSEData files contained in the PDB archive (ftp://ftp.wwpdb.org) are free of all copyright restrictions and made fully and freely available for both non-commercial and commercial use. Users of the data should attribute the original authors of that structural data.https://www.rcsb.org/pages/policies
- Springer Nature
- Wikidata
- Wikipedia
- PubChem
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlEnzyme Inhibitorshttps://www.ncbi.nlm.nih.gov/mesh/68004791
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 403659872https://pubchem.ncbi.nlm.nih.gov/substance/403659872
CONTENTS