Glyceryl dibehenate
PubChem CID
22477175
Molecular Formula
Synonyms
- Glyceryl dibehenate
- 99880-64-5
- docosanoic acid;propane-1,2,3-triol
- Docosanoic acid, diester with 1,2,3-propanetriol
- UNII-R8WTH25YS2
Molecular Weight
432.7 g/mol
Computed by PubChem 2.2 (PubChem release 2024.11.20)
Parent Compound
Component Compounds
Dates
- Create:2007-12-05
- Modify:2025-01-04
Chemical Structure Depiction
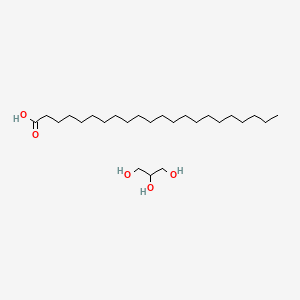
Conformer generation is disallowed since too flexible, mixture or salt
docosanoic acid;propane-1,2,3-triol
Computed by Lexichem TK 2.7.0 (PubChem release 2024.11.20)
InChI=1S/C22H44O2.C3H8O3/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20-21-22(23)24;4-1-3(6)2-5/h2-21H2,1H3,(H,23,24);3-6H,1-2H2
Computed by InChI 1.07.0 (PubChem release 2024.11.20)
BYNVYIUJKRRNNC-UHFFFAOYSA-N
Computed by InChI 1.07.0 (PubChem release 2024.11.20)
CCCCCCCCCCCCCCCCCCCCCC(=O)O.C(C(CO)O)O
Computed by OEChem 2.3.0 (PubChem release 2024.12.12)
C25H52O5
Computed by PubChem 2.2 (PubChem release 2024.11.20)
Compound: 1,2,3-Propanetriol, homopolymer, docosanoate
Compound: 1,2,3-Propanetriol, homopolymer, monodocosanoate
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
432.7 g/mol
Reference
Computed by PubChem 2.2 (PubChem release 2024.11.20)
Property Name
Hydrogen Bond Donor Count
Property Value
4
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2024.11.20)
Property Name
Hydrogen Bond Acceptor Count
Property Value
5
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2024.11.20)
Property Name
Rotatable Bond Count
Property Value
22
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2024.11.20)
Property Name
Exact Mass
Property Value
432.38147475 Da
Reference
Computed by PubChem 2.2 (PubChem release 2024.11.20)
Property Name
Monoisotopic Mass
Property Value
432.38147475 Da
Reference
Computed by PubChem 2.2 (PubChem release 2024.11.20)
Property Name
Topological Polar Surface Area
Property Value
98Ų
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2024.11.20)
Property Name
Heavy Atom Count
Property Value
30
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
276
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2024.11.20)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
2
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2021.10.14)
Cosmetic ingredients (Glyceryl Dibehenate) -> CIR (Cosmetic Ingredient Review)
Uv absorber; Emollient
S13 | EUCOSMETICS | Combined Inventory of Ingredients Employed in Cosmetic Products (2000) and Revised Inventory (2006) | DOI:10.5281/zenodo.2624118
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Same Parent, Connectivity Count
Same Parent, Exact Count
Mixtures, Components, and Neutralized Forms Count
Similar Compounds (2D)
Similar Conformers (3D)
Same Count
Cosmetic Ingredient Review Link
CIR ingredient: Glyceryl Dibehenate
Cosmetics -> Uv absorber; Emollient
S13 | EUCOSMETICS | Combined Inventory of Ingredients Employed in Cosmetic Products (2000) and Revised Inventory (2006) | DOI:10.5281/zenodo.2624118
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=BYNVYIUJKRRNNC-UHFFFAOYSA-N
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/Glycerol behenatehttps://commonchemistry.cas.org/detail?cas_rn=77538-19-3Glyceryl dibehenatehttps://commonchemistry.cas.org/detail?cas_rn=99880-64-51,2,3-Propanetriol, homopolymer, docosanoatehttps://commonchemistry.cas.org/detail?cas_rn=64366-79-61,2,3-Propanetriol, homopolymer, monodocosanoatehttps://commonchemistry.cas.org/detail?cas_rn=446021-95-0
- ChemIDplusGlyceryl dibehenatehttps://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0099880645ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- Cosmetic Ingredient Review (CIR)
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/NORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- WikidataGlyceryl dibehenatehttps://www.wikidata.org/wiki/Q126605230
- PubChem
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 387486511https://pubchem.ncbi.nlm.nih.gov/substance/387486511
CONTENTS
 CID 8215 (Behenic Acid)
CID 8215 (Behenic Acid) CID 753 (Glycerin)
CID 753 (Glycerin)