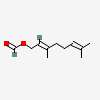
Geranyl formate
PubChem CID
5282109
Molecular Formula
Synonyms
- Geranyl formate
- 105-86-2
- 3,7-dimethylocta-2,6-dien-1-yl formate
- GERANIOL FORMATE
- FEMA No. 2514
Molecular Weight
182.26 g/mol
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Dates
- Create:2005-03-27
- Modify:2025-01-18
Description
(E)-geranyl formate is the formate ester of geraniol. It has a role as an alarm pheromone and a plant metabolite. It is functionally related to a geraniol.
Geranyl formate has been reported in Daphne odora, Elsholtzia ciliata, and other organisms with data available.
See also:  Neryl formate (annotation moved to).
Neryl formate (annotation moved to).
 Neryl formate (annotation moved to).
Neryl formate (annotation moved to).Chemical Structure Depiction
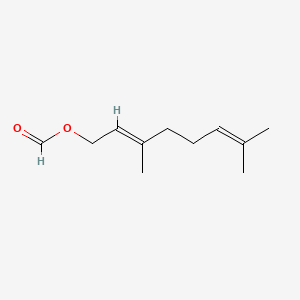
[(2E)-3,7-dimethylocta-2,6-dienyl] formate
Computed by Lexichem TK 2.7.0 (PubChem release 2021.10.14)
InChI=1S/C11H18O2/c1-10(2)5-4-6-11(3)7-8-13-9-12/h5,7,9H,4,6,8H2,1-3H3/b11-7+
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
FQMZVFJYMPNUCT-YRNVUSSQSA-N
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
CC(=CCC/C(=C/COC=O)/C)C
Computed by OEChem 2.3.0 (PubChem release 2024.12.12)
C11H18O2
Computed by PubChem 2.2 (PubChem release 2021.10.14)
2514
54
geranyl formate
- Geranyl formate
- 105-86-2
- 3,7-dimethylocta-2,6-dien-1-yl formate
- GERANIOL FORMATE
- FEMA No. 2514
- Geranyl methanoate
- 2,6-Octadien-1-ol, 3,7-dimethyl-, formate, (E)-
- NSC 21736
- [(2E)-3,7-dimethylocta-2,6-dienyl] formate
- trans-3,7-Dimethyl-2,6-octadien-1-ol formate
- trans-3,7-Dimethyl-2,6-octadien-1-yl formate
- Formic acid, geraniol ester
- 2,6-Octadien-1-ol, 3,7-dimethyl-, formate, (2E)-
- Formic acid, 3,7-dimethyl-2,6-octadienyl ester, (E)-
- 2,6-Octadien-1-ol, 3,7-dimethyl-, 1-formate, (2E)-
- DTXSID0047614
- CHEBI:31648
- 72O586X6ZI
- (E)-geranyl formate
- (E)-3,7-Dimethyl-2,6-octadienyl formate
- (E)-3,7-Dimethyl-2,6-octadien-1-ol formate
- WE(8:2(2E,6E)(3Me,7Me)/1:0)
- Geranyl formate (natural)
- (2E)-3,7-dimethylocta-2,6-dien-1-yl formate
- EINECS 203-339-4
- MFCD00021047
- BRN 1724191
- Geranylformiat
- UNII-72O586X6ZI
- AI3-01978
- NSC-21736
- 3,7-Dimethyl-2,6-octadienyl formate, (E)-
- 3,7-Dimethyl-2,6-octadien-1-yl methanoate, trans-
- 4-02-00-00035 (Beilstein Handbook Reference)
- GERANYL FORMATE [FCC]
- SCHEMBL226426
- SCHEMBL226427
- GERANYL FORMATE [FHFI]
- CHEMBL3182514
- DTXCID8027614
- Geranyl formate, >=95%, FCC
- Tox21_302545
- LMFA07010611
- AKOS015904157
- CS-W017095
- NCGC00256875-01
- BS-42294
- CAS-105-86-2
- 3,7-dimethyl-2,6-octadien-1-yl formate
- G0218
- NS00012498
- Q-201155
- Q15726045
- formic acid trans-3,7-dimethyl-oct-2,6-dien-1-ylester
- TRANS-2,6-DIMETHYL-2,6-OCTADIEN-8-YL METHANOATE
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
182.26 g/mol
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
XLogP3-AA
Property Value
3.5
Reference
Computed by XLogP3 3.0 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Donor Count
Property Value
0
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Acceptor Count
Property Value
2
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Rotatable Bond Count
Property Value
6
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Exact Mass
Property Value
182.130679813 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Monoisotopic Mass
Property Value
182.130679813 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Topological Polar Surface Area
Property Value
26.3 Ų
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Heavy Atom Count
Property Value
13
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
198
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
1
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
1
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2021.10.14)
colorless to pale yellow liquid/fresh, leafy, rose-like odour
1 ml in 3 ml 80% alcohol (in ethanol)
0.906-0.920
1.457-1.466
Standard non-polar
1290 , 1298 , 1284 , 1277 , 1279 , 1278.9 , 1281 , 1281 , 1281.4 , 1282 , 1285 , 1282 , 1278 , 1284 , 1284 , 1284 , 1283 , 1284 , 1284 , 1282 , 1283 , 1284 , 1281.6 , 1280 , 1290 , 1284 , 1282 , 1282 , 1280 , 1282 , 1280 , 1277 , 1279 , 1282
Semi-standard non-polar
1312 , 1298 , 1292 , 1298 , 1305 , 1304 , 1300 , 1305 , 1302 , 1322 , 1300 , 1234 , 1309 , 1329 , 1281 , 1298 , 1283 , 1300 , 1296 , 1299 , 1300 , 1301 , 1301
Standard polar
1686 , 1703 , 1721 , 1727 , 1698 , 1688 , 1717 , 1710 , 1691 , 1684 , 1695 , 1684 , 1665 , 1681 , 1709 , 1718 , 1684
Fatty Acyls [FA] -> Fatty esters [FA07] -> Short fatty esters [FA0710]
MoNA ID
MS Category
Experimental
MS Type
GC-MS
MS Level
MS1
Instrument
HITACHI M-80B
Instrument Type
EI-B
Ionization Mode
positive
Top 5 Peaks
69 99.99
41 50.19
68 21.34
136 11.54
39 10.09
License
CC BY-NC-SA
NIST Number
132450
Library
Main library
Total Peaks
124
m/z Top Peak
69
m/z 2nd Highest
41
m/z 3rd Highest
68
Thumbnail
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Same Connectivity Count
Same Parent, Connectivity Count
Same Parent, Exact Count
Mixtures, Components, and Neutralized Forms Count
Similar Compounds (2D)
Similar Conformers (3D)
 Neryl formate (annotation moved to)
Neryl formate (annotation moved to)JECFA Functional Classes
Flavouring Agent -> FLAVOURING_AGENT;
Flavoring Agents
Chemical Name
GERANYL FORMATE
Evaluation Year
2002
ADI
No safety concern at current levels of intake when used as a flavouring agent
Comments
Secondary components do not raise a safety concern
Report
Tox Monograph
EPA CPDat Chemical and Product Categories
The Chemical and Products Database, a resource for exposure-relevant data on chemicals in consumer products, Scientific Data, volume 5, Article number: 180125 (2018), DOI:10.1038/sdata.2018.125
Flavouring Agent -> FLAVOURING_AGENT; -> JECFA Functional Classes
Flavoring Agents -> JECFA Flavorings Index
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=FQMZVFJYMPNUCT-YRNVUSSQSA-N
The LOTUS Initiative for Open Natural Products Research: frozen dataset union wikidata (with metadata) | DOI:10.5281/zenodo.5794106
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/Geranyl formatehttps://commonchemistry.cas.org/detail?cas_rn=105-86-2
- ChemIDplusChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA DSSToxGeranyl formatehttps://comptox.epa.gov/dashboard/DTXSID0047614CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- ChEBI(E)-geranyl formatehttps://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:31648
- LOTUS - the natural products occurrence databaseLICENSEThe code for LOTUS is released under the GNU General Public License v3.0.https://lotus.nprod.net/Geranyl formatehttps://www.wikidata.org/wiki/Q15726045LOTUS Treehttps://lotus.naturalproducts.net/
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.html
- EPA Chemical and Products Database (CPDat)Geranyl formatehttps://comptox.epa.gov/dashboard/DTXSID0047614#exposureEPA CPDat Classificationhttps://www.epa.gov/chemical-research/chemical-and-products-database-cpdat
- Japan Chemical Substance Dictionary (Nikkaji)
- Joint FAO/WHO Expert Committee on Food Additives (JECFA)LICENSEPermission from WHO is not required for the use of WHO materials issued under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Intergovernmental Organization (CC BY-NC-SA 3.0 IGO) licence.https://www.who.int/about/policies/publishing/copyright
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.html
- KNApSAcK Species-Metabolite Database
- LIPID MAPSLipid Classificationhttps://www.lipidmaps.org/
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- NIST Mass Spectrometry Data CenterLICENSEData covered by the Standard Reference Data Act of 1968 as amended.https://www.nist.gov/srd/public-lawGeranyl formatehttp://www.nist.gov/srd/nist1a.cfm
- SpectraBaseGeranyl formatehttps://spectrabase.com/spectrum/K35HHaXQAlmGeraniol formatehttps://spectrabase.com/spectrum/JFqBKjly1GiGERANYL FORMATEhttps://spectrabase.com/spectrum/53QPBZ2nO7z3,7-DIMETHYL-2,6-OCTADIEN-1-OL, FORMATEhttps://spectrabase.com/spectrum/KEYR4HOow4G2,6-OCTADIEN-1-OL, 3,7-DIMETHYL-, FORMATEhttps://spectrabase.com/spectrum/J5lpmv7bOKv
- Metabolomics Workbench(E)-3,7-Dimethyl-2,6-octadienyl formatehttps://www.metabolomicsworkbench.org/data/StructureData.php?RegNo=4256
- Springer Nature
- SpringerMaterialsformic acid trans-3,7-dimethyl-oct-2,6-dien-1-ylesterhttps://materials.springer.com/substanceprofile/docs/smsid_kwwvmwscypcjmocy
- Thieme ChemistryLICENSEThe Thieme Chemistry contribution within PubChem is provided under a CC-BY-NC-ND 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc-nd/4.0/
- Wikidatageranyl formatehttps://www.wikidata.org/wiki/Q15726045
- PubChem
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlgeranyl formatehttps://www.ncbi.nlm.nih.gov/mesh/67572439
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/NORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- Consumer Product Information Database (CPID)LICENSECopyright (c) 2024 DeLima Associates. All rights reserved. Unless otherwise indicated, all materials from CPID are copyrighted by DeLima Associates. No part of these materials, either text or image may be used for any purpose other than for personal use. Therefore, reproduction, modification, storage in a retrieval system or retransmission, in any form or by any means, electronic, mechanical or otherwise, for reasons other than personal use, is strictly prohibited without prior written permission.https://www.whatsinproducts.com/contents/view/1/6Consumer Products Category Classificationhttps://www.whatsinproducts.com/
- EPA Chemicals under the TSCAEPA TSCA Classificationhttps://www.epa.gov/tsca-inventory
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 388353416https://pubchem.ncbi.nlm.nih.gov/substance/388353416
CONTENTS