FTase Inhibitor I
PubChem CID
132887
Molecular Formula
Synonyms
- 149759-96-6
- FTase Inhibitor I
- B 581
- B581
- B-581
Molecular Weight
470.7 g/mol
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Dates
- Create:2005-08-08
- Modify:2025-01-18
Description
B 581 is a dipeptide obtained from the tetrapeptide Cys-Val-Phe-Met by reduction of the amide carbonyl groups of the Cys and Val residues. It has a role as a peptidomimetic and an EC 2.5.1.58 (protein farnesyltransferase) inhibitor.
Chemical Structure Depiction
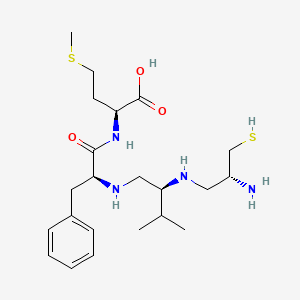
Conformer generation is disallowed since too flexible
SVG Image

Sequence
FM
(2S)-2-[[(2S)-2-[[(2S)-2-[[(2R)-2-amino-3-sulfanylpropyl]amino]-3-methylbutyl]amino]-3-phenylpropanoyl]amino]-4-methylsulfanylbutanoic acid
Computed by Lexichem TK 2.7.0 (PubChem release 2021.10.14)
InChI=1S/C22H38N4O3S2/c1-15(2)20(24-12-17(23)14-30)13-25-19(11-16-7-5-4-6-8-16)21(27)26-18(22(28)29)9-10-31-3/h4-8,15,17-20,24-25,30H,9-14,23H2,1-3H3,(H,26,27)(H,28,29)/t17-,18+,19+,20-/m1/s1
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
QISLMXIYRQCLIR-FUMNGEBKSA-N
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
CC(C)[C@@H](CN[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CCSC)C(=O)O)NC[C@H](CS)N
Computed by OEChem 2.3.0 (PubChem release 2024.12.12)
C22H38N4O3S2
Computed by PubChem 2.2 (PubChem release 2021.10.14)
- B 581
- B-581
- B581
- 149759-96-6
- FTase Inhibitor I
- B 581
- B581
- B-581
- CHEMBL91722
- CHEBI:83620
- L-Methionine, N-[(2S)-2-[[(2R)-2-amino-3-mercaptopropyl]amino]-3-methylbutyl]-L-phenylalanyl-
- (2S)-2-[[(2S)-2-[[(2S)-2-[[(2R)-2-amino-3-sulfanylpropyl]amino]-3-methylbutyl]amino]-3-phenylpropanoyl]amino]-4-methylsulfanylbutanoic acid
- L-Methionine, N-(N-(2-((2-amino-3-mercaptopropyl)amino)-3-methylbutyl)-L-phenylalanyl)-, (S-(R*,S*))-
- (S)-2-((S)-2-((S)-2-((R)-2-amino-3-mercaptopropylamino)-3-methylbutylamino)-3-phenylpropanamido)-4-(methylthio)butanoic acid
- CBiol_001875
- BSPBio_001466
- KBioGR_000186
- KBioSS_000186
- SCHEMBL2723960
- KBio2_000186
- KBio2_002754
- KBio2_005322
- KBio3_000371
- KBio3_000372
- DTXSID30164381
- Bio1_000161
- Bio1_000650
- Bio1_001139
- Bio2_000186
- Bio2_000666
- HMS1361J08
- HMS1791J08
- HMS1989J08
- HMS3402J08
- BDBM50059852
- MFCD00236999
- AKOS040744436
- B581, >95% (HPLC), solid
- IDI1_033936
- SMP2_000242
- NCGC00163425-01
- NCGC00163425-02
- NCGC00163425-03
- (S-(R*,S*))-N-(N-(2-((2-Amino-3-mercaptopropyl)amino)-3-methylbutyl)-L-phenylalanyl)-L-methionine
- DA-53417
- N-[2(S)-(2(R)-2-Amino-3-mercaptopropylamino)-3-methylbutyl]-L-phenylalanyl-L-methionine trifluoroacetate salt
- HY-128044
- CS-0095096
- J-008620
- BRD-K04877770-001-03-4
- Q27157026
- N-[2(S)-[2(R)-Amino-3-mercaptopropylamino]-3-methyl-butyl]-Phe-Met
- N-[2(S)-[2(R)-Amino-3-mercaptopropylamino]-3-methylbutyl]-Phe-Met-OH
- N-[(2S)-((2R)-2-Amino-3-mercaptopropylamino)-3-methylbutyl]-L-phenylalanyl-L-methionine
- (2S)-2-[(2S)-2-{[(2S)-2-{[(2R)-2-amino-3-sulfanylpropyl]amino}-3-methylbutyl]amino}-3-phenylpropanamido]-4-(methylsulfanyl)butanoic acid
- (2S)-2-[[(2S)-2-[[(2S)-2-[[(2R)-2-azaniumyl-3-sulfanylpropyl]amino]-3-methylbutyl]amino]-3-phenylpropanoyl]amino]-4-methylsulfanylbutanoate
- (S)-2-{(S)-2-[(S)-2-((R)-2-Amino-3-mercapto-propylamino)-3-methyl-butylamino]-3-phenyl-propionylamino}-4-methylsulfanyl-butyric acid
- N-[(2S)-2-{[(2R)-2-amino-3-sulfanylpropyl]amino}-3-methylbutyl]-L-phenylalanyl-L-methionine
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
470.7 g/mol
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
XLogP3-AA
Property Value
-0.1
Reference
Computed by XLogP3 3.0 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Donor Count
Property Value
6
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Acceptor Count
Property Value
8
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Rotatable Bond Count
Property Value
16
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Exact Mass
Property Value
470.23853343 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Monoisotopic Mass
Property Value
470.23853343 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Topological Polar Surface Area
Property Value
143 Ų
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Heavy Atom Count
Property Value
31
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
519
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
4
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
1
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2021.10.14)
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Same Connectivity Count
Same Parent, Connectivity Count
Same Parent, Exact Count
Mixtures, Components, and Neutralized Forms Count
Similar Compounds (2D)
Similar Conformers (3D)
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=QISLMXIYRQCLIR-FUMNGEBKSA-N
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/FTase inhibitor Ihttps://commonchemistry.cas.org/detail?cas_rn=149759-96-6
- ChemIDplusChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA DSSToxCompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- ChEBI
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- Comparative Toxicogenomics Database (CTD)LICENSEIt is to be used only for research and educational purposes. Any reproduction or use for commercial purpose is prohibited without the prior express written permission of NC State University.http://ctdbase.org/about/legal.jsp
- Japan Chemical Substance Dictionary (Nikkaji)
- PharosLICENSEData accessed from Pharos and TCRD is publicly available from the primary sources listed above. Please respect their individual licenses regarding proper use and redistribution.https://pharos.nih.gov/about(S)-2-{(S)-2-[(S)-2-((R)-2-Amino-3-mercapto-propylamino)-3-methyl-butylamino]-3-phenyl-propionylamino}-4-methylsulfanyl-butyric acidhttps://pharos.nih.gov/ligands/MM4CWS1FW4BG
- Springer Nature
- Therapeutic Target Database (TTD)
- Wikidata
- PubChem
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.html
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 389505155https://pubchem.ncbi.nlm.nih.gov/substance/389505155
CONTENTS