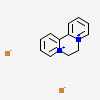
Diquat dibromide
- DIQUAT DIBROMIDE
- 85-00-7
- 9,10-Dihydro-8a,10a-diazoniaphenanthrene dibromide
- Aquacide
- Reglon
- Create:2005-08-08
- Modify:2025-01-11

- Dibromide, Diquat
- Diquat
- Diquat Dibromide
- DIQUAT DIBROMIDE
- 85-00-7
- 9,10-Dihydro-8a,10a-diazoniaphenanthrene dibromide
- Aquacide
- Reglon
- Reglox
- Deiquat
- Preeglone
- Reglone
- Weedtrine-D
- Diquat dibromide [ISO]
- Ethylene dipyridylium dibromide
- 6,7-Dihydrodipyrido[1,2-a:2',1'-c]pyrazine-5,8-diium bromide
- 6BDV3T272W
- 1,1'-Ethylene-2,2'-dipyridylium dibromide
- Detrone
- Dipyrido(1,2-a:2',1'-c)pyrazinediium, 6,7-dihydro-, dibromide
- Ortho-Diquat
- 34417-68-0
- Caswell No. 402
- Dipyrido[1,2-a:2',1'-c]pyrazinediium, 6,7-dihydro-, dibromide
- Reglon dibromide (USSR)
- Deiquat dibromide (USSR)
- 7,10-diazoniatricyclo[8.4.0.02,7]tetradeca-1(14),2,4,6,10,12-hexaene;dibromide
- Diquat dibromide 100 microg/mL in Water
- HSDB 1700
- NSC-116553
- EINECS 201-579-4
- PP 100
- EPA Pesticide Chemical Code 032201
- NSC 116553
- UNII-6BDV3T272W
- 1,1'-Ethylene 2,2'-dipyridylium dibromide
- 1,1'-Ethylene-2,2'-bipyridinium dibromide
- 1,1'-Ethylene-2,2'-bipyridylium dibromide
- 6,7-dihydrodipyrido[1,2-a:2',1'-c]pyrazinediium dibromide
- 1,1'-Aethylen-2,2'-bipyridinium-dibromid [German]
- 6,7-Dihydrodipyrido(1,2-a:2',1'-c)pyrazinedium dibromide
- 1,1'-Aethylen-2,2'-bipyridinium-dibromid
- 9,10-Dihydro-8a,10a-diazoniaphenanthrene(1,1'-ethylene-2,2'-bipyridylium)dibromide
- SCHEMBL52886
- DIQUAT DIBROMIDE [MI]
- DIQUAT DIBROMIDE [HSDB]
- CHEMBL1599022
- DTXSID3024075
- DIQUAT DIBROMIDE [MART.]
- ODPOAESBSUKMHD-UHFFFAOYSA-L
- Dipyrido(1,2-a;2',1'-c)pyrazinediium, 6,7-dihydro-, dibromide
- MFCD00078635
- AKOS015902672
- Dipyrido(1,2-a:2',1'-c)pyrazinediium, 6,7-dihydro-, labeled with carbon-14, dibromide
- NCGC00163714-01
- AS-15492
- HY-122984
- CS-0090817
- NS00078917
- G88708
- A917748
- Q910903
- 9,10-DIHYDRO-8A,10A-DIAZOPHENANTHRENE DIBROMIDE
- 6,7-DIHYDRO-DIPYRIDO(1,2-A:2',1-C)PYRAZINEDIIUM DIBROMIDE
- DIPYRIDO(1,2-A:2',1'-C)PYRAZINEDIIUM, 6,7-DIHYDRO-, BROMIDE (1:2)
 Diquat (annotation moved to)
Diquat (annotation moved to)(See general first aid procedures)
Eye: Irrigate immediately - If this chemical contacts the eyes, immediately wash (irrigate) the eyes with large amounts of water, occasionally lifting the lower and upper lids. Get medical attention immediately.
Skin: Water flush immediately - If this chemical contacts the skin, immediately flush the contaminated skin with water. If this chemical penetrates the clothing, immediately remove the clothing and flush the skin with water. Get medical attention promptly.
Breathing: Respiratory support
Swallow: Medical attention immediately - If this chemical has been swallowed, get medical attention immediately.
N.D.
See: IDLH INDEX
(See personal protection and sanitation codes)
Skin: Prevent skin contact - Wear appropriate personal protective clothing to prevent skin contact.
Eyes: Prevent eye contact - Wear appropriate eye protection to prevent eye contact.
Wash skin: When contaminated
Remove: When wet or contaminated
Change: Daily - Workers whose clothing may have become contaminated should change into uncontaminated clothing before leaving the work premises.
Provide: Quick drench - Facilities for quickly drenching the body should be provided within the immediate work area for emergency use where there is a possibility of exposure. [Note: It is intended that these facilities provide a sufficient quantity or flow of water to quickly remove the substance from any body areas likely to be exposed. The actual determination of what constitutes an adequate quick drench facility depends on the specific circumstances. In certain instances, a deluge shower should be readily available, whereas in others, the availability of water from a sink or hose could be considered adequate.]
Hazard Traits - Developmental Toxicity; Ocular Toxicity
Authoritative List - CA MCLs; CWA 303(d)
Report - if used as a fragrance or flavor ingredient
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=ODPOAESBSUKMHD-UHFFFAOYSA-L
- California Safe Cosmetics Program (CSCP) Product Database
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.html
- ChemIDplusDiquat dibromide [ISO]https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000085007Dipyrido(1,2-a:2',1'-c)pyrazinediium, 6,7-dihydro-, labeled with carbon-14, dibromidehttps://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0034417680ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA DSSToxDiquat dibromidehttps://comptox.epa.gov/dashboard/DTXSID3024075CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingDIQUAT DIBROMIDEhttps://gsrs.ncats.nih.gov/ginas/app/beta/substances/6BDV3T272W
- Risk Assessment Information System (RAIS)LICENSEThis work has been sponsored by the U.S. Department of Energy (DOE), Office of Environmental Management, Oak Ridge Operations (ORO) Office through a joint collaboration between United Cleanup Oak Ridge LLC (UCOR), Oak Ridge National Laboratory (ORNL), and The University of Tennessee, Ecology and Evolutionary Biology, The Institute for Environmental Modeling (TIEM). All rights reserved.https://rais.ornl.gov/Diquat dibromidehttps://rais.ornl.gov/cgi-bin/tools/TOX_search
- EPA Chemical and Products Database (CPDat)EPA CPDat Classificationhttps://www.epa.gov/chemical-research/chemical-and-products-database-cpdat
- EPA Pesticide Ecotoxicity Database
- SpectraBaseDIQUAT DIBROMIDEhttps://spectrabase.com/spectrum/ApzzZc0i9nl6,7-DIHYDRODIPYRIDO[1,2-a:2',1'-c]PYRAZINEDIIUM DIBROMIDEhttps://spectrabase.com/spectrum/58TntpYqNWy
- Springer Nature
- The National Institute for Occupational Safety and Health (NIOSH)LICENSEThe information provided using CDC Web site is only intended to be general summary information to the public. It is not intended to take the place of either the written law or regulations.https://www.cdc.gov/Other/disclaimer.htmlDiquat (Diquat dibromide)https://www.cdc.gov/niosh/npg/npgd0243.html
- Toxin and Toxin Target Database (T3DB)LICENSET3DB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (T3DB) and the original publication.http://www.t3db.ca/downloadsDiquat dibromidehttp://www.t3db.ca/toxins/T3D4498
- USGS Columbia Environmental Research CenterLICENSEhttps://www.usgs.gov/foia
- Wikidatadiquat dibromidehttps://www.wikidata.org/wiki/Q910903
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlDefoliants, Chemicalhttps://www.ncbi.nlm.nih.gov/mesh/68003678
- PubChem
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- CAMEO ChemicalsLICENSECAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data.https://cameochemicals.noaa.gov/help/reference/terms_and_conditions.htm?d_f=falseCAMEO Chemical Reactivity Classificationhttps://cameochemicals.noaa.gov/browse/react
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/NORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- Consumer Product Information Database (CPID)LICENSECopyright (c) 2024 DeLima Associates. All rights reserved. Unless otherwise indicated, all materials from CPID are copyrighted by DeLima Associates. No part of these materials, either text or image may be used for any purpose other than for personal use. Therefore, reproduction, modification, storage in a retrieval system or retransmission, in any form or by any means, electronic, mechanical or otherwise, for reasons other than personal use, is strictly prohibited without prior written permission.https://www.whatsinproducts.com/contents/view/1/6Consumer Products Category Classificationhttps://www.whatsinproducts.com/
- EPA Substance Registry ServicesEPA SRS List Classificationhttps://sor.epa.gov/sor_internet/registry/substreg/LandingPage.do
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 403432890https://pubchem.ncbi.nlm.nih.gov/substance/403432890

 CID 260 (Hydrobromic Acid)
CID 260 (Hydrobromic Acid)