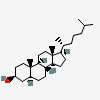
Cholestanol
- Dihydrocholesterol
- 80-97-7
- beta-Cholestanol
- 5alpha-Cholestan-3beta-ol
- CHOLESTANOL
- Create:2005-07-19
- Modify:2025-01-18
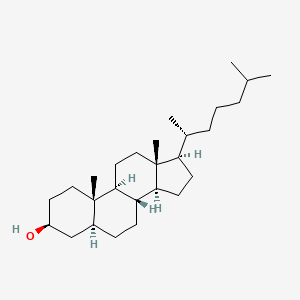
- 5 alpha Cholestan 3 alpha ol
- 5 alpha Cholestan 3 beta ol
- 5 alpha-Cholestan-3 alpha-ol
- 5 alpha-Cholestan-3 beta-ol
- 5 beta Cholestan 3 beta ol
- 5 beta-Cholestan-3 alpha-ol
- 5 beta-Cholestan-3 beta-ol
- beta Cholestanol
- beta-Cholestan-3 beta-ol, 5
- beta-Cholestanol
- beta-ol, 5 beta-Cholestan-3
- Cholestan 3 ol
- Cholestan-3-ol
- Cholestanol
- Cholestanol, (3alpha, 5beta)-Isomer
- Coprostanol
- Coprosterol
- Dihydrocholesterol
- Dihydrocholesterol
- 80-97-7
- beta-Cholestanol
- 5alpha-Cholestan-3beta-ol
- CHOLESTANOL
- Zymostanol
- 5alpha-Cholestanol
- Cholestan-3-ol, (3beta,5alpha)-
- Dihydrocholesterin
- Cholestan-3beta-ol
- 5a-Cholestan-3b-ol
- 3beta-Cholestanol
- Cholestanol (VAN)
- 5-alpha-Cholestan-3-beta-ol
- 3beta-Hydroxy-5alpha-cholestane
- 5a-Cholestanol
- 5|A-Cholestan-3|A-ol
- Cholestan-3b-ol
- MFCD00066413
- (5alpha)-cholestan-3beta-ol
- 3b-Hydroxycholestane
- 5a-Dihydrocholesterol
- UNII-8M308U816E
- CHEBI:86570
- CHOLESTANOL [MI]
- EINECS 201-315-8
- 3b-Hydroxy-5a-cholestane
- NSC 18188
- (3b,5a)-Cholestan-3-ol
- CHOLESTANOL [USP-RS]
- beta Cholestanol
- (3S,5S,8R,9S,10S,13R,14S,17R)-10,13-dimethyl-17-[(2R)-6-methylheptan-2-yl]-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-ol
- (3beta,5alpha)-cholestan-3-ol
- Cholestan 3 ol
- DTXSID40883258
- NSC-18188
- 8M308U816E
- 29466-38-4
- 5.alpha.-Cholestanol
- CHOLESTAN-3-OL,(3.BETA.,5.ALPHA.)-
- Epicholestanol
- 5 beta Cholestan 3 beta ol
- 5 beta-Cholestan-3 beta-ol
- CHOLESTANOL (USP-RS)
- 5 alpha Cholestan 3 beta ol
- 5 alpha-Cholestan-3 beta-ol
- 5 beta-Cholestan-3 alpha-ol
- Cholestan-3-ol, (3.beta.,5.alpha.)-
- 5 alpha Cholestan 3 alpha ol
- 5 alpha-Cholestan-3 alpha-ol
- beta-Cholestan-3 beta-ol, 5
- beta-ol, 5 beta-Cholestan-3
- Dihydrocholestenol
- 5.alpha.-Cholestan-3.beta.-ol
- Cholestan-3-ol, (3b,5a)-
- Cholestanol, (3alpha, 5beta)-Isomer
- Cholestan-3.beta.-ol
- 5?-cholestan-3?-ol
- 3beta-Hydroxycholestane
- (+)-Dihydrocholesterol
- 5alpha-Dihydrocholesterol
- Cholestan-3-ol, (3alpha)-
- DTXCID401474016
- Cholestan-3-ol, (3.beta.)- #
- (3S,5S,8R,9S,10S,13R,14S,17R)-10,13-dimethyl-17-((2R)-6-methylheptan-2-yl)-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta(a)phenanthren-3-ol
- 10,13-dimethyl-17-(6-methylheptan-2-yl)-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta(a)phenanthren-3-ol
- 10,13-dimethyl-17-(6-methylheptan-2-yl)-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-ol
- 5-Cholestan-3-ol
- bmse000541
- SCHEMBL133951
- CHEMBL1289436
- 5 alpha -Cholestan-3 beta -ol
- DIHYDROCHOLESTEROL [INCI]
- DTXCID901022802
- 27409-41-2
- LMST01010077
- s5123
- 5alpha-Cholestan-3beta-ol, >=95%
- Cholestane, 3beta-hydroxy-, 5alpha-
- AKOS016036214
- CCG-268506
- AS-58118
- CHOLESTAN-3-OL,(3BETA,5ALPHA)-
- 1ST001398
- HY-107819
- CS-0030696
- Dihydrocholesterol;5-Cholestanol;NSC 18188
- D94638
- Q27159255
- 1814DD66-70FE-4610-86FB-FA8455101768
- Cholestanol, United States Pharmacopeia (USP) Reference Standard
- (3S,5S,8R,9S,10S,13R,14S,17R)-17-((R)-1,5-dimethylhexyl)-10,13-dimethylhexadecahydrocyclopenta[a]phenanthren-3-ol
215.0 99.99
233.0 87.70
165.0 52.30
234.0 46.70
107.0 45.90
388.0 99.99
234.0 79.20
233.0 47.60
44.0 36.80
215.0 32.50
215.0 1
233.0 0.88
165.0 0.52
234.0 0.47
107.0 0.46
388.0 1
234.0 0.79
233.0 0.48
44.0 0.37
215.0 0.33
240.908873 100
98.975727 76.95
116.986111 63.79
222.898451 51.36
61.011358 39.44
306.919324 100
98.975744 90.93
59.061088 72.10
116.986105 70.23
264.908932 66.93
215 999
233 877
165 523
234 467
107 459
388 999
234 792
233 476
44 368
215 325
- Brain
- Eye Lens
- Fibroblasts
- Liver
- Extracellular
- Membrane
Not Classified
Reported as not meeting GHS hazard criteria by 1 of 1 companies. For more detailed information, please visit ECHA C&L website.
Silke Matysik, Caroline Ivanne Le Roy, Gerhard Liebisch, Sandrine Paule Claus. Metabolomics of fecal samples: A practical consideration. Trends in Food Science & Technology. Vol. 57, Part B, Nov. 2016, p.244-255: http://www.sciencedirect.com/science/article/pii/S0924224416301984
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=QYIXCDOBOSTCEI-QCYZZNICSA-N
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/
- ChemIDplusChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA Chemicals under the TSCACholestan-3-ol, (3.beta.,5.alpha.)-https://www.epa.gov/chemicals-under-tscaEPA TSCA Classificationhttps://www.epa.gov/tsca-inventory
- EPA DSSTox(+)-Dihydrocholesterolhttps://comptox.epa.gov/dashboard/DTXSID40883258CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-notice5-α-cholestan-3-β-olhttps://echa.europa.eu/substance-information/-/substanceinfo/100.001.1975-α-cholestan-3-β-ol (EC: 201-315-8)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/2552
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingDIHYDROCHOLESTEROLhttps://gsrs.ncats.nih.gov/ginas/app/beta/substances/8M308U816E
- Human Metabolome Database (HMDB)LICENSEHMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications.http://www.hmdb.ca/citing5alpha-Cholestanolhttp://www.hmdb.ca/metabolites/HMDB0000908HMDB0000908_cms_27302https://hmdb.ca/metabolites/HMDB0000908#spectra
- CCSbaseCCSbase Classificationhttps://ccsbase.net/
- ChEBI(5alpha)-cholestan-3beta-olhttps://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:86570
- LOTUS - the natural products occurrence databaseLICENSEThe code for LOTUS is released under the GNU General Public License v3.0.https://lotus.nprod.net/Dihydrocholesterolhttps://www.wikidata.org/wiki/Q27159255LOTUS Treehttps://lotus.naturalproducts.net/
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- Comparative Toxicogenomics Database (CTD)LICENSEIt is to be used only for research and educational purposes. Any reproduction or use for commercial purpose is prohibited without the prior express written permission of NC State University.http://ctdbase.org/about/legal.jsp
- Crystallography Open Database (COD)LICENSEAll data in the COD and the database itself are dedicated to the public domain and licensed under the CC0 License. Users of the data should acknowledge the original authors of the structural data.https://creativecommons.org/publicdomain/zero/1.0/
- EPA Chemical and Products Database (CPDat)Cholestan-3-ol, (3.beta.,5.alpha.)-https://comptox.epa.gov/dashboard/DTXSID40883258#exposureEPA CPDat Classificationhttps://www.epa.gov/chemical-research/chemical-and-products-database-cpdat
- FooDBLICENSEFooDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (FooDB) and the original publication.https://foodb.ca/aboutCholestanolhttps://foodb.ca/compounds/FDB012122
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- NIST Mass Spectrometry Data CenterLICENSEData covered by the Standard Reference Data Act of 1968 as amended.https://www.nist.gov/srd/public-lawCholestanolhttp://www.nist.gov/srd/nist1a.cfm
- SpectraBase5α-cholestan-3β-olhttps://spectrabase.com/spectrum/BkrQCaLqwi25a-Cholestan-3b-ol (8CI)https://spectrabase.com/spectrum/91E15fIWcls5α-cholestan-3β-olhttps://spectrabase.com/spectrum/B9WM7pNrLLT5α-cholestan-3β-olhttps://spectrabase.com/spectrum/HiK8MugwXqO5α-Cholestan-3β-olhttps://spectrabase.com/spectrum/Lhpk86G8aWW5α-cholestan-3β-olhttps://spectrabase.com/spectrum/9nwTqSbmhIK5α-cholestan-3β-olhttps://spectrabase.com/spectrum/Gr6jyg9DXls5α-cholestan-3β-olhttps://spectrabase.com/spectrum/DjoRW5gLJ6I5α-cholestan-3β-olhttps://spectrabase.com/spectrum/2LFAqg0jpTk5α-cholestan-3β-olhttps://spectrabase.com/spectrum/5LXiGpD29Wj5α-cholestan-3β-olhttps://spectrabase.com/spectrum/IUn3Qvlpg6Z5α-cholestan-3β-olhttps://spectrabase.com/spectrum/ImjbvvhGMNw5α-cholestan-3β-olhttps://spectrabase.com/spectrum/GuFz39a5qgU
- Japan Chemical Substance Dictionary (Nikkaji)
- Kruve Lab, Ionization & Mass Spectrometry, Stockholm University5-alpha-cholestan-3-beta-ol
- LIPID MAPSLipid Classificationhttps://www.lipidmaps.org/
- Natural Product Activity and Species Source (NPASS)(3S,5S,10S,13R,17R)-17-((R)-1,5-Dimethyl-Hexyl)-10,13-Dimethyl-Hexadecahydro-Cyclopenta[A]Phenanthren-3-Olhttps://bidd.group/NPASS/compound.php?compoundID=NPC71460
- MarkerDBLICENSEThis work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.https://markerdb.ca/5-alpha-Cholestanolhttps://markerdb.ca/chemicals/483
- MassBank Europe
- Metabolomics WorkbenchDihydrocholesterolhttps://www.metabolomicsworkbench.org/data/StructureData.php?RegNo=34389
- Nature Chemical Biology
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/NORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- Rhea - Annotated Reactions DatabaseLICENSERhea has chosen to apply the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0/). This means that you are free to copy, distribute, display and make commercial use of the database in all legislations, provided you credit (cite) Rhea.https://www.rhea-db.org/help/license-disclaimer
- Springer Nature
- Thieme ChemistryLICENSEThe Thieme Chemistry contribution within PubChem is provided under a CC-BY-NC-ND 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc-nd/4.0/
- Wikidatadihydrocholesterolhttps://www.wikidata.org/wiki/Q27159255
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlCholestanolhttps://www.ncbi.nlm.nih.gov/mesh/68004083
- PubChem
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- EPA Substance Registry ServicesEPA SRS List Classificationhttps://sor.epa.gov/sor_internet/registry/substreg/LandingPage.do
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 403469697https://pubchem.ncbi.nlm.nih.gov/substance/403469697
- NCBI