Chloranocryl
PubChem CID
16560
Molecular Formula
Synonyms
- Chloranocryl
- 2164-09-2
- DICRYL
- N-(3,4-Dichlorophenyl)methacrylamide
- Licryl
Molecular Weight
230.09 g/mol
Computed by PubChem 2.2 (PubChem release 2024.11.20)
Dates
- Create:2005-03-27
- Modify:2025-01-11
Description
Chloranocryl is an anilide.
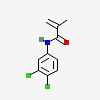
Chemical Structure Depiction

N-(3,4-dichlorophenyl)-2-methylprop-2-enamide
Computed by Lexichem TK 2.7.0 (PubChem release 2024.11.20)
InChI=1S/C10H9Cl2NO/c1-6(2)10(14)13-7-3-4-8(11)9(12)5-7/h3-5H,1H2,2H3,(H,13,14)
Computed by InChI 1.07.0 (PubChem release 2024.11.20)
VCBRBUKGTWLJOB-UHFFFAOYSA-N
Computed by InChI 1.07.0 (PubChem release 2024.11.20)
CC(=C)C(=O)NC1=CC(=C(C=C1)Cl)Cl
Computed by OEChem 2.3.0 (PubChem release 2024.12.12)
C10H9Cl2NO
Computed by PubChem 2.2 (PubChem release 2024.11.20)
- (Z)-isomer of dichloromaleic acid
- 2,3-dichloro-2-butenedioic acid, (Z)-isomer
- DCMA
- dichloromaleic acid
- dichloromaleic acid, Z-isomer
- Chloranocryl
- 2164-09-2
- DICRYL
- N-(3,4-Dichlorophenyl)methacrylamide
- Licryl
- Chloranocryl [ISO]
- 3',4'-Dichloro-2-methylacrylanilide
- DCMA
- Niagara 4556
- N-(3,4-dichlorophenyl)-2-methylprop-2-enamide
- Caswell No. 329
- N-(3,4-Dichlorophenyl)-2-methyl-2-propenamide
- Acrylanilide, 3',4'-dichloro-2-methyl-
- FMC 4556
- NIA 4556
- 3',4'-Dichloro-2-methacrylanilide
- Methacrylic acid 3,4-dichloroanilide
- Z411Q69NZW
- 2-Propenamide, N-(3,4-dichlorophenyl)-2-methyl-
- DTXSID2020424
- 3,4'-Dichloro-2-methylacrylanilide
- DICRYL [MI]
- N 4,556
- EPA Pesticide Chemical Code 032601
- 3,4-Dichloranilid kyseliny methakrylove
- BRN 2806300
- UNII-Z411Q69NZW
- DTXCID00424
- AI3-26034
- alpha-Methylacrylic acid, 3,4-dichloroanilide
- CHEBI:82173
- 3,4-Dichloranilid kyseliny methakrylove [Czech]
- .alpha.-Methylacrylic acid, 3,4-dichloroanilide
- (Z)-N-(3,4-dichlorophenyl)-2-methylprop-2-enimidic acid
- CAS-2164-09-2
- SCHEMBL132197
- CHEMBL3184602
- Tox21_201516
- Tox21_302753
- AKOS015888392
- NCGC00249059-01
- NCGC00256546-01
- NCGC00259066-01
- NS00015386
- N-(3,4-Dichlorophenyl)-2-methylacrylamide #
- C19046
- Q27155782
- 2-Propenamide, N-(3,4-dichlorophenyl)-2-methyl-(9CI)
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
230.09 g/mol
Reference
Computed by PubChem 2.2 (PubChem release 2024.11.20)
Property Name
XLogP3
Property Value
4.2
Reference
Computed by XLogP3 3.0 (PubChem release 2024.11.20)
Property Name
Hydrogen Bond Donor Count
Property Value
1
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2024.11.20)
Property Name
Hydrogen Bond Acceptor Count
Property Value
1
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2024.11.20)
Property Name
Rotatable Bond Count
Property Value
2
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2024.11.20)
Property Name
Exact Mass
Property Value
229.0061193 Da
Reference
Computed by PubChem 2.2 (PubChem release 2024.11.20)
Property Name
Monoisotopic Mass
Property Value
229.0061193 Da
Reference
Computed by PubChem 2.2 (PubChem release 2024.11.20)
Property Name
Topological Polar Surface Area
Property Value
29.1 Ų
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2024.11.20)
Property Name
Heavy Atom Count
Property Value
14
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
242
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2024.11.20)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
1
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2021.10.14)
Standard non-polar
1869.4
NIST Number
60496
Library
Main library
Total Peaks
161
m/z Top Peak
69
m/z 2nd Highest
41
m/z 3rd Highest
39
Thumbnail
NIST Number
59666
Library
Replicate library
Total Peaks
139
m/z Top Peak
41
m/z 2nd Highest
69
m/z 3rd Highest
39
Thumbnail
Accession ID
Authors
Elapavalore, A.; Kondić, T.; Singh, R.; Schymanski, E.
Instrument
Q Exactive Orbitrap (Thermo Scientific)
Instrument Type
LC-ESI-QFT
MS Level
MS2
Ionization Mode
NEGATIVE
Ionization
ESI
Collision Energy
15
Fragmentation Mode
HCD
Column Name
Acquity BEH C18 1.7um, 2.1x150mm (Waters)
Retention Time
17.863 min
Precursor m/z
227.9988
Precursor Adduct
[M-H]-
Top 5 Peaks
227.9988 999
55.0189 11
156.0455 8
225.9834 7
185.9518 4
License
CC BY
Reference
Elapavalore, A.; Kondić, T.; et al., Adding Open Spectral Data to MassBank and PubChem Using Open Source Tools to Support Non-Targeted Exposomics of Mixtures (submitted).
Accession ID
Authors
Elapavalore, A.; Kondić, T.; Singh, R.; Schymanski, E.
Instrument
Q Exactive Orbitrap (Thermo Scientific)
Instrument Type
LC-ESI-QFT
MS Level
MS2
Ionization Mode
NEGATIVE
Ionization
ESI
Collision Energy
30
Fragmentation Mode
HCD
Column Name
Acquity BEH C18 1.7um, 2.1x150mm (Waters)
Retention Time
17.863 min
Precursor m/z
227.9988
Precursor Adduct
[M-H]-
Top 5 Peaks
227.9989 999
55.0189 49
156.0456 31
225.9834 26
185.9519 23
License
CC BY
Reference
Elapavalore, A.; Kondić, T.; et al., Adding Open Spectral Data to MassBank and PubChem Using Open Source Tools to Support Non-Targeted Exposomics of Mixtures (submitted).
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Mixtures, Components, and Neutralized Forms Count
Similar Compounds (2D)
Similar Conformers (3D)
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=VCBRBUKGTWLJOB-UHFFFAOYSA-N
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/
- ChemIDplusChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA DSSToxChloranocrylhttps://comptox.epa.gov/dashboard/DTXSID2020424CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- ChEBI
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.html
- Japan Chemical Substance Dictionary (Nikkaji)
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.html
- MassBank Europe
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- NIST Mass Spectrometry Data CenterLICENSEData covered by the Standard Reference Data Act of 1968 as amended.https://www.nist.gov/srd/public-law2-Propenamide, N-(3,4-dichlorophenyl)-2-methyl-http://www.nist.gov/srd/nist1a.cfm
- SpectraBase2-Propenamide, N-(3,4-dichlorophenyl)-2-methyl-https://spectrabase.com/spectrum/EPHf1wnRfmI2-Propenamide, N-(3,4-dichlorophenyl)-2-methyl-https://spectrabase.com/spectrum/DgpNuvlUfOE
- Springer Nature
- SpringerMaterialsN-(3,4-dichlorophenyl)-2-methyl-2-propenamidehttps://materials.springer.com/substanceprofile/docs/smsid_irufzfymefjrmifv
- Wikidatachloranocrylhttps://www.wikidata.org/wiki/Q27155782
- PubChem
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmldichloromaleic acidhttps://www.ncbi.nlm.nih.gov/mesh/67062588
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/NORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- EPA Substance Registry ServicesEPA SRS List Classificationhttps://sor.epa.gov/sor_internet/registry/substreg/LandingPage.do
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 403444885https://pubchem.ncbi.nlm.nih.gov/substance/403444885
CONTENTS