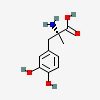
(2R)-2-amino-3-(3,4-dihydroxyphenyl)-2-methylpropanoic acid
- 2799-15-7
- D-alpha-Methyl DOPA
- D-Methyldopa
- (2R)-2-amino-3-(3,4-dihydroxyphenyl)-2-methylpropanoic acid
- Methyldopa, D-
- Create:2005-07-08
- Modify:2025-01-11
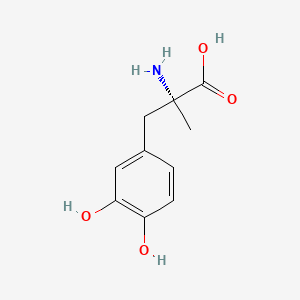

- 2799-15-7
- D-alpha-Methyl DOPA
- D-Methyldopa
- (2R)-2-amino-3-(3,4-dihydroxyphenyl)-2-methylpropanoic acid
- Methyldopa, D-
- (R)-2-amino-3-(3,4-dihydroxyphenyl)-2-methylpropanoic acid
- 8K91SZC8W9
- D-a-Methyl DOPA
- L-Methyldopa
- (2R)-2-amino-3-(3,4-dihydroxyphenyl)-2-methyl-propanoic acid
- (+)-alpha-Methyldopa
- UNII-8K91SZC8W9
- 3-Hydroxy-alpha-Methyl-D-Tyrosine
- CAS-555-30-6
- D-(3,4-Dihydroxyphenyl)-2-methylalanine
- Lopac-M-129
- D- alpha -Methyl DOPA
- Lopac-M-7277
- D-3-(3,4-Dihydroxyphenyl)-2-methylalanine
- D-.ALPHA.-METHYLDOPA
- SCHEMBL1321546
- CHEMBL1452561
- METHYLDOPA ANHYDROUS, D-
- (+)-.ALPHA.-METHYLDOPA
- IMT-002
- AKOS030242221
- NCGC00015631-01
- NCGC00015631-02
- NCGC00016510-01
- NCGC00016510-02
- NCGC00016510-03
- NCGC00094724-01
- NCGC00094724-02
- DB-179449
- METHYLDOPA IMPURITY D [EP IMPURITY]
- NS00080410
- D-TYROSINE, 3-HYDROXY-.ALPHA.-METHYL-
- EN300-225307
- Alanine, 3-(3,4-dihydroxyphenyl)-2-methyl-, D-
- J-016939
- Q27270669
- D-.ALPHA.-METHYL-3-(3,4-DIHYDROXYPHENYL)ALANINE
- Z1508927176
- (2R)-2-amino-3-(3,4-dihydroxyphenyl)-2-methylpropanoicacid
◉ Summary of Use during Lactation
Because of the low levels of methyldopa in breastmilk, amounts ingested by the infant are small and would not be expected to cause any adverse effects in breastfed infants. No special precautions are required.
◉ Effects in Breastfed Infants
No acute or long-term adverse effects were reported in any 15 infants ranging in age from less than 1 week to 8 weeks of age whose mothers were taking oral methyldopa 0.25 to 1.5 grams daily.
◉ Effects on Lactation and Breastmilk
Methyldopa can increase serum prolactin and has caused galactorrhea. The maternal prolactin level in a mother with established lactation may not affect her ability to breastfeed.
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=CJCSPKMFHVPWAR-SNVBAGLBSA-N
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/D-α-Methyldopahttps://commonchemistry.cas.org/detail?cas_rn=2799-15-7
- ChemIDplus(+)-alpha-Methyldopahttps://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0002799157ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.html
- Drugs and Lactation Database (LactMed)
- Japan Chemical Substance Dictionary (Nikkaji)
- Springer Nature
- Therapeutic Target Database (TTD)
- WikidataD-methyldopahttps://www.wikidata.org/wiki/Q27270669
- PubChem
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/NORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 388553154https://pubchem.ncbi.nlm.nih.gov/substance/388553154
- NCBI