Corynebactin
PubChem CID
125349
Molecular Formula
Synonyms
- Corynebactin
- Bacillibactin
- 95536-41-7
- 36PG6K45CP
- 95536-04-2
Molecular Weight
882.8 g/mol
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Dates
- Create:2005-06-24
- Modify:2025-01-18
Description
Corynebactin is a crown compound that is enterobactin in which the pro-R hydrogens at positions 2, 6 and 10 of the trilactone backbone are replaced by methyl groups, and in which a glycine spacer separates the trilactone backbone from each of the catecholamide arms. It is the endogenous siderophore of Bacillus subtilis, used for the acquisition of iron. It has a role as a metabolite and a bacterial metabolite. It is a crown compound, a member of catechols and a macrocyclic lactone.
Corynebactin has been reported in Bacillus subtilis, Corynebacterium glutamicum, and other organisms with data available.
Chemical Structure Depiction
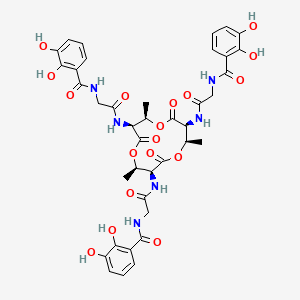
Conformer generation is disallowed since too many atoms, too flexible
IUPAC Condensed
Bz(2,3-diOH)-Gly-Thr(1)-(3).Bz(2,3-diOH)-Gly-Thr(2)-(1).Bz(2,3-diOH)-Gly-Thr(3)-(2)
N-[2-[[(2R,3S,6R,7S,10R,11S)-7,11-bis[[2-[(2,3-dihydroxybenzoyl)amino]acetyl]amino]-2,6,10-trimethyl-4,8,12-trioxo-1,5,9-trioxacyclododec-3-yl]amino]-2-oxoethyl]-2,3-dihydroxybenzamide
Computed by Lexichem TK 2.7.0 (PubChem release 2021.10.14)
InChI=1S/C39H42N6O18/c1-16-28(43-25(49)13-40-34(55)19-7-4-10-22(46)31(19)52)37(58)62-18(3)30(45-27(51)15-42-36(57)21-9-6-12-24(48)33(21)54)39(60)63-17(2)29(38(59)61-16)44-26(50)14-41-35(56)20-8-5-11-23(47)32(20)53/h4-12,16-18,28-30,46-48,52-54H,13-15H2,1-3H3,(H,40,55)(H,41,56)(H,42,57)(H,43,49)(H,44,50)(H,45,51)/t16-,17-,18-,28+,29+,30+/m1/s1
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
RCQTVEFBFUNTGM-BDVHUIKKSA-N
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
C[C@@H]1[C@@H](C(=O)O[C@@H]([C@@H](C(=O)O[C@@H]([C@@H](C(=O)O1)NC(=O)CNC(=O)C2=C(C(=CC=C2)O)O)C)NC(=O)CNC(=O)C3=C(C(=CC=C3)O)O)C)NC(=O)CNC(=O)C4=C(C(=CC=C4)O)O
Computed by OEChem 2.3.0 (PubChem release 2024.12.12)
C39H42N6O18
Computed by PubChem 2.2 (PubChem release 2021.10.14)
95536-04-2
corynebactin
- Corynebactin
- Bacillibactin
- 95536-41-7
- 36PG6K45CP
- 95536-04-2
- N,N',N''-{[(2R,3S,6R,7S,10R,11S)-2,6,10-trimethyl-4,8,12-trioxo-1,5,9-trioxacyclododecane-3,7,11-triyl]tris[imino(2-oxoethane-2,1-diyl)]}tris(2,3-dihydroxybenzamide)
- N-[2-[[(2R,3S,6R,7S,10R,11S)-7,11-bis[[2-[(2,3-dihydroxybenzoyl)amino]acetyl]amino]-2,6,10-trimethyl-4,8,12-trioxo-1,5,9-trioxacyclododec-3-yl]amino]-2-oxoethyl]-2,3-dihydroxybenzamide
- 9-Gln-beta-lipotropin
- beta-Lipotropin, gln(9)-
- 9-Glutamine-beta-lipotropin
- beta-Lipotropin, glutamine(9)-
- N-(2-(((2R,3S,6R,7S,10R,11S)-7,11-BIS((2-((2,3-DIHYDROXYBENZOYL)AMINO)ACETYL)AMINO)-2,6,10-TRIMETHYL-4,8,12-TRIOXO-1,5,9-TRIOXACYCLODODEC-3-YL)AMINO)-2-OXOETHYL)-2,3-DIHYDROXYBENZAMIDE
- UNII-36PG6K45CP
- SCHEMBL18913548
- CHEBI:31432
- DTXSID101045559
- 2,3-dihydroxybenzoate-glycine-threonine trimeric ester
- Q14931586
- L-THREONINE, N-(2,3-DIHYDROXYBENZOYL)GLYCYL-, TRIMOL. (2->2'),(2'->2''),(2''->2)-TRILACTONE
- N,N',N''-((((2R,3S,6R,7S,10R,11S)-2,6,10-Trimethyl-4,8,12-trioxo-1,5,9-trioxacyclododecane-3,7,11-triyl)tris(azanediyl))tris(2-oxoethane-2,1-diyl))tris(2,3-dihydroxybenzamide)
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
882.8 g/mol
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
XLogP3-AA
Property Value
1.6
Reference
Computed by XLogP3 3.0 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Donor Count
Property Value
12
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Acceptor Count
Property Value
18
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Rotatable Bond Count
Property Value
12
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Exact Mass
Property Value
882.25555851 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Monoisotopic Mass
Property Value
882.25555851 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Topological Polar Surface Area
Property Value
375 Ų
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Heavy Atom Count
Property Value
63
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
1510
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
6
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
1
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2021.10.14)
MoNA ID
MS Category
Experimental
MS Type
LC-MS
MS Level
MS2
Precursor Type
[M+H]+
Precursor m/z
883.265
Instrument
qTof
Ionization Mode
positive
Top 5 Peaks
883.264893 100
690.226624 57.18
884.264343 43.89
691.224915 20.39
885.264282 15.57
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Same Connectivity Count
Same Parent, Connectivity Count
Similar Compounds (2D)
Similar Conformers (3D)
Same Count
The LOTUS Initiative for Open Natural Products Research: frozen dataset union wikidata (with metadata) | DOI:10.5281/zenodo.5794106
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/
- ChemIDplusbeta-Lipotropin, gln(9)-https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0095536042ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA DSSToxBacillibactinhttps://comptox.epa.gov/dashboard/DTXSID101045559CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- ChEBI
- LOTUS - the natural products occurrence databaseLICENSEThe code for LOTUS is released under the GNU General Public License v3.0.https://lotus.nprod.net/Corynebactinhttps://www.wikidata.org/wiki/Q14931586LOTUS Treehttps://lotus.naturalproducts.net/
- Japan Chemical Substance Dictionary (Nikkaji)
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.html
- Natural Product Activity and Species Source (NPASS)
- The Natural Products AtlasLICENSEThe Natural Products Atlas is licensed under a Creative Commons Attribution 4.0 International License.https://www.npatlas.org/termsThe Natural Products Atlas Classificationhttps://www.npatlas.org/
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- Metabolomics Workbench
- Rhea - Annotated Reactions DatabaseLICENSERhea has chosen to apply the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0/). This means that you are free to copy, distribute, display and make commercial use of the database in all legislations, provided you credit (cite) Rhea.https://www.rhea-db.org/help/license-disclaimer
- Wikidatacorynebactinhttps://www.wikidata.org/wiki/Q14931586
- WikipediaBacillibactinhttps://en.wikipedia.org/wiki/Bacillibactin
- PubChem
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlcorynebactinhttps://www.ncbi.nlm.nih.gov/mesh/67044318
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- NCBI
CONTENTS