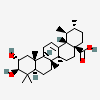
Corosolic acid
PubChem CID
6918774
Molecular Formula
Synonyms
- Corosolic acid
- 4547-24-4
- Glucosol
- Corsolic acid
- 2alpha-Hydroxyursolic acid
Molecular Weight
472.7 g/mol
Computed by PubChem 2.2 (PubChem release 2024.11.20)
Dates
- Create:2006-07-28
- Modify:2025-01-18
Description
Corosolic acid is a triterpenoid. It has a role as a metabolite.
Corosolic acid has been reported in Salvia miltiorrhiza, Rosa laevigata, and other organisms with data available.
See also: Lagerstroemia speciosa leaf (part of).
Chemical Structure Depiction
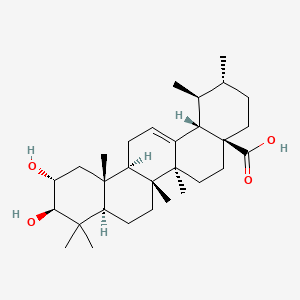
(1S,2R,4aS,6aR,6aS,6bR,8aR,10R,11R,12aR,14bS)-10,11-dihydroxy-1,2,6a,6b,9,9,12a-heptamethyl-2,3,4,5,6,6a,7,8,8a,10,11,12,13,14b-tetradecahydro-1H-picene-4a-carboxylic acid
Computed by Lexichem TK 2.7.0 (PubChem release 2024.11.20)
InChI=1S/C30H48O4/c1-17-10-13-30(25(33)34)15-14-28(6)19(23(30)18(17)2)8-9-22-27(5)16-20(31)24(32)26(3,4)21(27)11-12-29(22,28)7/h8,17-18,20-24,31-32H,9-16H2,1-7H3,(H,33,34)/t17-,18+,20-,21+,22-,23+,24+,27+,28-,29-,30+/m1/s1
Computed by InChI 1.07.0 (PubChem release 2024.11.20)
HFGSQOYIOKBQOW-ZSDYHTTISA-N
Computed by InChI 1.07.0 (PubChem release 2024.11.20)
C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(C[C@H]([C@@H](C5(C)C)O)O)C)C)[C@@H]2[C@H]1C)C)C(=O)O
Computed by OEChem 2.3.0 (PubChem release 2024.12.12)
C30H48O4
Computed by PubChem 2.2 (PubChem release 2024.11.20)
- 2alpha-hydroxyursoloic acid
- corosolic acid
- Glucosol
- Corosolic acid
- 4547-24-4
- Glucosol
- Corsolic acid
- 2alpha-Hydroxyursolic acid
- 2-alpha-Hydroxyursolic acid
- Corosolic-acid
- UNII-AMX2I57A98
- AMX2I57A98
- Colosolic acid
- CHEMBL391533
- CHEBI:67895
- DTXSID70904142
- (2alpha,3beta)-2,3-Dihydroxyurs-12-en-28-oic acid
- COROSOLIC ACID (USP-RS)
- COROSOLIC ACID [USP-RS]
- Colosic acid
- (2a,3b)-2,3-dihydroxy-urs-12-en-28-oic acid
- 2alpha-hydroxyursoloic acid
- Colosolic acid; Corsolic acid; Glucosol
- MFCD06794973
- Corosolic acid (Standard)
- MLS000563488
- SCHEMBL335528
- HY-N0280R
- Ursolic acid (2-alpha-hydroxy-)
- DTXCID501332027
- Urs-12-en-28-oic acid, 2,3-dihydroxy-, (2alpha,3beta)-
- Corosolic acid, analytical standard
- HY-N0280
- BDBM50222205
- s9041
- 2-.ALPHA.-HYDROXYURSOLIC ACID
- AKOS032948365
- CCG-269470
- CS-3798
- NCGC00247602-01
- (1S,2R,4aS,6aR,6aS,6bR,8aR,10R,11R,12aR,14bS)-10,11-dihydroxy-1,2,6a,6b,9,9,12a-heptamethyl-2,3,4,5,6,6a,7,8,8a,10,11,12,13,14b-tetradecahydro-1H-picene-4a-carboxylic acid
- AC-33935
- BS-42423
- SMR000232346
- 2alpha,3beta-dihydroxyurs-12-en-28-oic acid
- COROSOLIC ACID (CONSTITUENT OF BANABA LEAF)
- Q-100487
- Q5172335
- BRD-K27885593-001-06-8
- (2alpha,3beta)-2,3-Dihydroxy-urs-12-en-28-oic Acid
- 2alpha,2beta-dihydroxy-18beta-ursan-12-ene-28-oic acid
- COROSOLIC ACID (CONSTITUENT OF BANABA LEAF) [DSC]
- Corosolic acid from Lagerstroemia speciosa, >=98% (HPLC)
- Corosolic acid, United States Pharmacopeia (USP) Reference Standard
- URS-12-EN-28-OIC ACID, 2,3-DIHYDROXY-, (2.ALPHA.,3.BETA.)-
- (1S,2R,4aS,6aS,6bR,8aR,10R,11R,12aR,12bR,14bS)-10,11-dihydroxy-1,2,6a,6b,9,9,12a-heptamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-icosahydropicene-4a-carboxylic acid
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
472.7 g/mol
Reference
Computed by PubChem 2.2 (PubChem release 2024.11.20)
Property Name
XLogP3-AA
Property Value
6.4
Reference
Computed by XLogP3 3.0 (PubChem release 2024.11.20)
Property Name
Hydrogen Bond Donor Count
Property Value
3
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2024.11.20)
Property Name
Hydrogen Bond Acceptor Count
Property Value
4
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2024.11.20)
Property Name
Rotatable Bond Count
Property Value
1
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2024.11.20)
Property Name
Exact Mass
Property Value
472.35526001 Da
Reference
Computed by PubChem 2.2 (PubChem release 2024.11.20)
Property Name
Monoisotopic Mass
Property Value
472.35526001 Da
Reference
Computed by PubChem 2.2 (PubChem release 2024.11.20)
Property Name
Topological Polar Surface Area
Property Value
77.8 Ų
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2024.11.20)
Property Name
Heavy Atom Count
Property Value
34
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
907
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2024.11.20)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
11
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
1
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2021.10.14)
Accession ID
Authors
Plant Biology, The Noble Foundation, Ardmore, OK, US/Dennis Fine, Daniel Wherritt, and Lloyd Sumner
Instrument
Bruker impact HD
Instrument Type
LC-ESI-QTOF
MS Level
MS2
Ionization Mode
NEGATIVE
Ionization
ESI
Collision Energy
20 eV
Column Name
Waters Acquity BEH C18 1.7um x 2.1 x 150 mm
Retention Time
1407 sec
Precursor m/z
471.3528
Precursor Adduct
[M-H]-
Top 5 Peaks
471.3528 999
472.3553 252
473.3589 18
474.358 1
License
CC BY-NC-SA
Accession ID
Authors
Plant Biology, The Noble Foundation, Ardmore, OK, US/Dennis Fine, Daniel Wherritt, and Lloyd Sumner
Instrument
Bruker impact HD
Instrument Type
LC-ESI-QTOF
MS Level
MS2
Ionization Mode
NEGATIVE
Ionization
ESI
Collision Energy
10 eV
Column Name
Waters Acquity BEH C18 1.7um x 2.1 x 150 mm
Retention Time
1407 sec
Precursor m/z
471.3524
Precursor Adduct
[M-H]-
Top 5 Peaks
471.3524 999
472.3553 265
473.3567 19
471.4522 2
474.3593 2
License
CC BY-NC-SA
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Same Connectivity Count
Same Parent, Connectivity Count
Same Parent, Exact Count
Mixtures, Components, and Neutralized Forms Count
Similar Compounds (2D)
Similar Conformers (3D)
Lagerstroemia speciosa leaf (part of)
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=HFGSQOYIOKBQOW-ZSDYHTTISA-N
The LOTUS Initiative for Open Natural Products Research: frozen dataset union wikidata (with metadata) | DOI:10.5281/zenodo.5794106
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/Corosolic acidhttps://commonchemistry.cas.org/detail?cas_rn=4547-24-4
- ChemIDplusChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA DSSToxCorosolic acidhttps://comptox.epa.gov/dashboard/DTXSID70904142CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-noticeUrs-12-en-28-oic acid, 2,3-dihydroxy-, (2α,3β)-https://echa.europa.eu/substance-information/-/substanceinfo/100.125.730
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- ChEBI
- LOTUS - the natural products occurrence databaseLICENSEThe code for LOTUS is released under the GNU General Public License v3.0.https://lotus.nprod.net/Corosolic acidhttps://www.wikidata.org/wiki/Q5172335LOTUS Treehttps://lotus.naturalproducts.net/
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- Comparative Toxicogenomics Database (CTD)LICENSEIt is to be used only for research and educational purposes. Any reproduction or use for commercial purpose is prohibited without the prior express written permission of NC State University.http://ctdbase.org/about/legal.jspcorosolic acidhttps://ctdbase.org/detail.go?type=chem&acc=C113861
- Therapeutic Target Database (TTD)Corosolic acidhttps://idrblab.net/ttd/data/drug/details/D0F9RO
- FooDBLICENSEFooDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (FooDB) and the original publication.https://foodb.ca/aboutUrsolic acid (2-alpha-hydroxy-)https://foodb.ca/compounds/FDB097386Verbascosidehttps://foodb.ca/compounds/FDB097390
- Human Metabolome Database (HMDB)LICENSEHMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications.http://www.hmdb.ca/citingUrsolic acid (2-alpha-hydroxy-)http://www.hmdb.ca/metabolites/HMDB0304746
- Japan Chemical Substance Dictionary (Nikkaji)
- Natural Product Activity and Species Source (NPASS)
- MassBank Europe
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- Metabolomics Workbench
- PharosLICENSEData accessed from Pharos and TCRD is publicly available from the primary sources listed above. Please respect their individual licenses regarding proper use and redistribution.https://pharos.nih.gov/aboutCorosolic acidhttps://pharos.nih.gov/ligands/X5XG53ZWLS5D
- SpectraBase2-ALPHA-HYDROXY-URSOLIC-ACIDhttps://spectrabase.com/spectrum/LLM5VizT5aA2-ALPHA-HYDROXY-URSOLIC-ACIDhttps://spectrabase.com/spectrum/JcHOE65uVNW(1S,2R,4aS,6aR,6aS,6bR,8aR,10R,11R,12aR,14bS)-1,2,6a,6b,9,9,12a-heptamethyl-10,11-bis(oxidanyl)-2,3,4,5,6,6a,7,8,8a,10,11,12,13,14b-tetradecahydro-1H-picene-4a-carboxylic acidhttps://spectrabase.com/spectrum/B0OPI3Rc1zM
- Wikidatacorosolic acidhttps://www.wikidata.org/wiki/Q5172335
- WikipediaCorosolic acidhttps://en.wikipedia.org/wiki/Corosolic_acid
- PubChem
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlcorosolic acidhttps://www.ncbi.nlm.nih.gov/mesh/67113861
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 393977861https://pubchem.ncbi.nlm.nih.gov/substance/393977861
CONTENTS