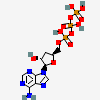
Cordycepin triphosphate
PubChem CID
65562
Molecular Formula
Synonyms
- Cordycepin triphosphate
- 3'-Deoxyadenosine 5'-triphosphate
- 73-04-1
- 3'-deoxyadenosine-5'-triphosphate
- CoTP
Molecular Weight
491.18 g/mol
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Dates
- Create:2005-06-24
- Modify:2025-01-18
Description
Cordycepin triphosphate is a purine ribonucleoside 5'-triphosphate. It has a role as an antimetabolite, an antifungal agent, an antineoplastic agent and an antiviral agent. It is functionally related to a cordycepin.
Cordycepin Triphosphate is the triphosphate salt of cordycepin, a purine nucleoside antimetabolite and antibiotic isolated from the fungus Cordyceps militaris with potential antineoplastic, antioxidant, and anti-inflammatory activities. Cordycepin is an inhibitor of polyadenylation, activates AMP-activated protein kinase (AMPK), and reduces mammalian target of rapamycin (mTOR) signaling, which may result in both the induction of tumor cell apoptosis and a decrease in tumor cell proliferation. mTOR, a serine/threonine kinase belonging to the phosphatidylinositol 3-kinase (PI3K)-related kinase (PIKK) family, plays an important role in the PI3K/AKT/mTOR signaling pathway that regulates cell growth and proliferation, and its expression or activity is frequently dysregulated in human cancers.
See also: Cordycepin 5'-triphosphate sodium (annotation moved to).
Chemical Structure Depiction
IUPAC Condensed
P-P-P-Ade-3dRibf
Sequence
N
IUPAC
5'-triphospho-3'-deoxy-adenosine
[[(2S,4R,5R)-5-(6-aminopurin-9-yl)-4-hydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl] phosphono hydrogen phosphate
Computed by Lexichem TK 2.7.0 (PubChem release 2021.10.14)
InChI=1S/C10H16N5O12P3/c11-8-7-9(13-3-12-8)15(4-14-7)10-6(16)1-5(25-10)2-24-29(20,21)27-30(22,23)26-28(17,18)19/h3-6,10,16H,1-2H2,(H,20,21)(H,22,23)(H2,11,12,13)(H2,17,18,19)/t5-,6+,10+/m0/s1
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
NLIHPCYXRYQPSD-BAJZRUMYSA-N
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
C1[C@H](O[C@H]([C@@H]1O)N2C=NC3=C(N=CN=C32)N)COP(=O)(O)OP(=O)(O)OP(=O)(O)O
Computed by OEChem 2.3.0 (PubChem release 2024.12.12)
C10H16N5O12P3
Computed by PubChem 2.2 (PubChem release 2021.10.14)
- 3'-deoxyadenosine 5'-triphosphate
- cordycepin triphosphate
- Cordycepin triphosphate
- 3'-Deoxyadenosine 5'-triphosphate
- 73-04-1
- 3'-deoxyadenosine-5'-triphosphate
- CoTP
- 3'-dATP
- 71997-32-5
- 3'-deoxyadenosine triphosphate
- [[(2S,4R,5R)-5-(6-aminopurin-9-yl)-4-hydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl] phosphono hydrogen phosphate
- CHEBI:52316
- 59P84ZU54H
- 3'-deoxyadenosine 5'-(tetrahydrogen triphosphate)
- Adenosine 5'-(tetrahydrogen triphosphate), 3'-deoxy-
- 1xfv
- 3AT
- UNII-59P84ZU54H
- ATP,3'-deoxy
- 3'dATP
- 3'-DEOXY-ATP
- SCHEMBL250251
- CORDYCEPIN, TRIPHOSPHATE
- CHEMBL480329
- BDBM86487
- CORDYCEPIN 5'-TRIPHOSPHATE
- DTXSID501014467
- CORDYCEPIN TRIPHOSPHATE [MI]
- DB01860
- 3-Deoxyadenosine 5-triphosphate sodium salt
- NS00070905
- ADENOSINE, 3'-DEOXY-, 5'-TRIPHOSPHATE
- 3'-DEOXYRIBOFURANOSYLADENINE 5'-TRIPHOSPHATE
- ADENOSINE, 3'-DEOXY-, 5'-(TETRAHYDROGEN TRIPHOSPHATE)
- ((2S,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-hydroxytetrahydrofuran-2-yl)methyl tetrahydrogen triphosphate
- [[(2S,4R,5R)-5-(6-aminopurin-9-yl)-4-hydroxy-tetrahydrofuran-2-yl]methoxy-hydroxy-phosphoryl] phosphono hydrogen phosphate
- {[(2S,4R,5R)-5-(6-Aminopurin-9-yl)-4-hydroxyoxolan-2-yl]methoxy}(hydroxyphosphoryl) dihydrogen phosphate
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
491.18 g/mol
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
XLogP3
Property Value
-4.5
Reference
Computed by XLogP3 3.0 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Donor Count
Property Value
6
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Acceptor Count
Property Value
16
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Rotatable Bond Count
Property Value
8
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Exact Mass
Property Value
491.00083196 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Monoisotopic Mass
Property Value
491.00083196 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Topological Polar Surface Area
Property Value
259 Ų
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Heavy Atom Count
Property Value
30
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
769
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
3
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
1
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2021.10.14)
Pharmaceuticals -> Listed in ZINC15
S55 | ZINC15PHARMA | Pharmaceuticals from ZINC15 | DOI:10.5281/zenodo.3247749
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Same Connectivity Count
Same Parent, Connectivity Count
Same Parent, Exact Count
Mixtures, Components, and Neutralized Forms Count
Similar Compounds (2D)
Similar Conformers (3D)
Cordycepin 5'-triphosphate sodium (annotation moved to)
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=NLIHPCYXRYQPSD-BAJZRUMYSA-N
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/3′-Deoxyadenosine 5′-(tetrahydrogen triphosphate)https://commonchemistry.cas.org/detail?cas_rn=73-04-1
- ChemIDplus3'-Deoxyadenosine 5'-triphosphatehttps://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000073041ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- DrugBankLICENSECreative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode)https://www.drugbank.ca/legal/terms_of_useCordycepin Triphosphatehttps://www.drugbank.ca/drugs/DB01860
- EPA DSSTox3'-Deoxyadenosine 5'-triphosphatehttps://comptox.epa.gov/dashboard/DTXSID501014467CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingCORDYCEPIN TRIPHOSPHATEhttps://gsrs.ncats.nih.gov/ginas/app/beta/substances/59P84ZU54H
- ChEBICordycepin triphosphatehttps://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:52316
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- Japan Chemical Substance Dictionary (Nikkaji)
- Metabolomics WorkbenchCordycepin triphosphatehttps://www.metabolomicsworkbench.org/data/StructureData.php?RegNo=58226
- Natural Product Activity and Species Source (NPASS)
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/Cordycepin triphosphateNORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- Protein Data Bank in Europe (PDBe)
- RCSB Protein Data Bank (RCSB PDB)LICENSEData files contained in the PDB archive (ftp://ftp.wwpdb.org) are free of all copyright restrictions and made fully and freely available for both non-commercial and commercial use. Users of the data should attribute the original authors of that structural data.https://www.rcsb.org/pages/policies
- Springer Nature
- WikidataCordycepin Triphosphatehttps://www.wikidata.org/wiki/Q27092987
- PubChem
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.html3'-deoxyadenosine 5'-triphosphatehttps://www.ncbi.nlm.nih.gov/mesh/67011746
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 403525024https://pubchem.ncbi.nlm.nih.gov/substance/403525024
- NCBI
CONTENTS