Cenisertib
PubChem CID
11569967
Molecular Formula
Synonyms
- Cenisertib
- 871357-89-0
- AS703569
- Cenisertib [INN]
- AS-703569
Molecular Weight
451.5 g/mol
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Dates
- Create:2006-10-26
- Modify:2025-01-18
Description
Cenisertib is an aurora kinase inhibitor.
Cenisertib is a water-soluble, synthetic small molecule with potential antineoplastic activity. Cenisertib selectively binds to and inhibits aurora kinases (AKs), a family of serine-threonine kinases which are important regulators of cell division and proliferation, and which are overexpressed in certain types of cancer. Inhibition of aurora kinases inhibits cell division and proliferation and induces apoptosis in tumor cells overexpressing AKs.
Chemical Structure Depiction
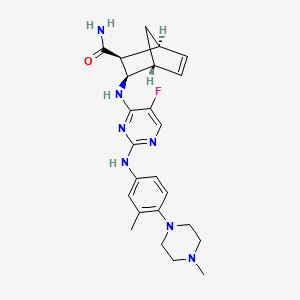
(1S,2S,3R,4R)-3-[[5-fluoro-2-[3-methyl-4-(4-methylpiperazin-1-yl)anilino]pyrimidin-4-yl]amino]bicyclo[2.2.1]hept-5-ene-2-carboxamide
Computed by Lexichem TK 2.7.0 (PubChem release 2021.10.14)
InChI=1S/C24H30FN7O/c1-14-11-17(5-6-19(14)32-9-7-31(2)8-10-32)28-24-27-13-18(25)23(30-24)29-21-16-4-3-15(12-16)20(21)22(26)33/h3-6,11,13,15-16,20-21H,7-10,12H2,1-2H3,(H2,26,33)(H2,27,28,29,30)/t15-,16+,20+,21-/m1/s1
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
KSOVGRCOLZZTPF-QMKUDKLTSA-N
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
CC1=C(C=CC(=C1)NC2=NC=C(C(=N2)N[C@@H]3[C@@H]4C[C@H]([C@@H]3C(=O)N)C=C4)F)N5CCN(CC5)C
Computed by OEChem 2.3.0 (PubChem release 2024.12.12)
C24H30FN7O
Computed by PubChem 2.2 (PubChem release 2021.10.14)
871357-89-0
- AS703569
- MSC1992371A
- R763 compound
- Cenisertib
- 871357-89-0
- AS703569
- Cenisertib [INN]
- AS-703569
- R-763
- UNII-5277GPA358
- (1S,2S,3R,4R)-3-((5-fluoro-2-((3-methyl-4-(4-methylpiperazin-1-yl)phenyl)amino)pyrimidin-4-yl)amino)bicyclo[2.2.1]hept-5-ene-2-carboxamide
- 5277GPA358
- AS 703569
- CENISERTIB [WHO-DD]
- (1S,2S,3R,4R)-3-[[5-fluoro-2-[3-methyl-4-(4-methylpiperazin-1-yl)anilino]pyrimidin-4-yl]amino]bicyclo[2.2.1]hept-5-ene-2-carboxamide
- CHEMBL1614709
- R 763
- Aurora kinase inhibitor as703569
- compound 2 [PMID: 22695126]
- enantiomer E1 [WO2005118544A2]
- R763
- compound 60a [WO2005118544A2]
- Bicyclo(2.2.1)hept-5-ene-2-carboxamide, 3-((5-fluoro-2-((3-methyl-4-(4-methyl-1-piperazinyl)phenyl)amino)-4-pyrimidinyl)amino)-, (1S,2S,3R,4R)-
- compound 2 (PMID: 22695126)
- (1S,2S,3R,4R)-3-((5-FLUORO-2-((3-METHYL-4-(4-METHYLPIPERAZIN-1-YL)PHENYL)AMINO)PYRIMIDIN-4-YL)AMINO)BICYCLO(2.2.1)HEPT-5-ENE-2-CARBOXAMIDE
- compound 60a (WO2005118544A2)
- enantiomer E1 (WO2005118544A2)
- cenisertibum
- DTXSID20236147
- (1S,2S,3R,4R)-3-((5-fluoro-2-(3-methyl-4-(4-methylpiperazin-1-yl)anilino)pyrimidin-4-yl)amino)bicyclo(2.2.1)hept-5-ene-2-carboxamide
- (1S,2S,3R,4R)-3-[(5-fluoro-2-{[3-methyl-4-(4-methylpiperazin-1-yl)phenyl]amino}pyrimidin-4-yl)amino]bicyclo[2.2.1]hept-5-ene-2-carboxamide
- AS-703569;R-763
- SCHEMBL400147
- GTPL9927
- DTXCID20158638
- KSOVGRCOLZZTPF-QMKUDKLTSA-N
- BDBM50389967
- NSC763930
- AKOS040741531
- DB06347
- NSC-763930
- HY-13072
- MS-28200
- DB-344506
- CS-0003175
- G14148
- Q27260973
- (1R,2R,3S,4S)-N4-(3-Aminocarbonylbicyclo[2.2.1]hept-5-en-2-yl)-5-fluoro-N2-[3-methyl-4-(4-methlypiperazin-1-yl)phenyl]-2,4-pyrimidinediamine
- (1R,2R,3S,4S)-N4-(3-Aminocarbonylbicyclo[2.2.1]hept-5-en-2-yl)-5-fluoro-N2-[3-methyl-4-(4-methylpiperazin-1-yl)phenyl]-2,4-pyrimidinediamine
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
451.5 g/mol
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
XLogP3-AA
Property Value
3.2
Reference
Computed by XLogP3 3.0 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Donor Count
Property Value
3
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Acceptor Count
Property Value
8
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Rotatable Bond Count
Property Value
6
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Exact Mass
Property Value
451.24958677 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Monoisotopic Mass
Property Value
451.24958677 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Topological Polar Surface Area
Property Value
99.4 Ų
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Heavy Atom Count
Property Value
33
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
730
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
4
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
1
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2021.10.14)
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Same Connectivity Count
Same Parent, Connectivity Count
Same Parent, Exact Count
Mixtures, Components, and Neutralized Forms Count
Similar Compounds (2D)
Similar Conformers (3D)
PubMed Count
Investigated for use/treatment in solid tumors, leukemia (myeloid), myelodysplastic syndrome, and cancer/tumors (unspecified).
R763 is a highly potent and specific inhibitor of Aurora kinase, which has been shown to block proliferation and trigger apoptosis (cell death) in several tumor cell lines including cervical, colon, lung, pancreas and prostate. The over-expression of Aurora kinase can cause cells to rapidly develop an abnormal number of chromosomes. Elevated levels of Aurora kinase are frequently associated with various human cancers and inhibition of this enzyme disrupts cell division and promotes apoptosis. [Rigel Pharmaceuticals Press Release] A probable target for R763 is Aurora kinase A (serine/threonine protein kinase 6).
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=KSOVGRCOLZZTPF-QMKUDKLTSA-N
- BindingDBLICENSEAll data curated by BindingDB staff are provided under the Creative Commons Attribution 3.0 License (https://creativecommons.org/licenses/by/3.0/us/).https://www.bindingdb.org/rwd/bind/info.jspAminopyrimidine (Scaffold VI)https://www.bindingdb.org/rwd/bind/chemsearch/marvin/MolStructure.jsp?monomerid=50389967
- Drug Gene Interaction database (DGIdb)LICENSEThe data used in DGIdb is all open access and where possible made available as raw data dumps in the downloads section.http://www.dgidb.org/downloadsCENISERTIBhttps://www.dgidb.org/drugs/ncit:C64540
- DrugBankLICENSECreative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode)https://www.drugbank.ca/legal/terms_of_useCenisertibhttps://www.drugbank.ca/drugs/DB06347
- IUPHAR/BPS Guide to PHARMACOLOGYLICENSEThe Guide to PHARMACOLOGY database is licensed under the Open Data Commons Open Database License (ODbL) https://opendatacommons.org/licenses/odbl/. Its contents are licensed under a Creative Commons Attribution-ShareAlike 4.0 International License (http://creativecommons.org/licenses/by-sa/4.0/)https://www.guidetopharmacology.org/about.jsp#licenseGuide to Pharmacology Target Classificationhttps://www.guidetopharmacology.org/targets.jsp
- Therapeutic Target Database (TTD)
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- ChemIDplusChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- ClinicalTrials.govLICENSEThe ClinicalTrials.gov data carry an international copyright outside the United States and its Territories or Possessions. Some ClinicalTrials.gov data may be subject to the copyright of third parties; you should consult these entities for any additional terms of use.https://clinicaltrials.gov/ct2/about-site/terms-conditions#Use
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- Metabolomics Workbench
- PharosLICENSEData accessed from Pharos and TCRD is publicly available from the primary sources listed above. Please respect their individual licenses regarding proper use and redistribution.https://pharos.nih.gov/about
- Wikidatacenisertibhttps://www.wikidata.org/wiki/Q27260973
- PubChemPFAS and Fluorinated Compounds in PubChemhttps://gitlab.com/uniluxembourg/lcsb/eci/pubchem-docs/-/raw/main/pfas-tree/PFAS_Tree.pdf?inline=false
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlMSC1992371Ahttps://www.ncbi.nlm.nih.gov/mesh/2002768
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 390671938https://pubchem.ncbi.nlm.nih.gov/substance/390671938
CONTENTS

