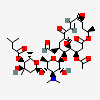
Carbomycin A
PubChem CID
5287879
Molecular Formula
Synonyms
- CARBOMYCIN
- Carbomycin A
- Magnamycin
- Magnamycin A
- Deltamycin A4
Molecular Weight
842.0 g/mol
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Dates
- Create:2005-11-29
- Modify:2025-01-11
Description
Carbomycin, also called magnamycin, is crystalline macrolide antibiotic. This antibacterial is obtained from Streptomyces halstedii and it presents a large inhibitory effect against Gram-positive bacteria and some Mycoplasma strains. The structure of carbomycin was generated in 1957 by Robert Woodward and later modified in 1965.
See also: Carbomycin (annotation moved to).
Chemical Structure Depiction
Conformer generation is disallowed since too many atoms
[(2S,3S,4R,6S)-6-[(2R,3S,4R,5R,6S)-6-[[(1S,3R,7R,8S,9S,10R,12R,14E,16S)-7-acetyloxy-8-methoxy-3,12-dimethyl-5,13-dioxo-10-(2-oxoethyl)-4,17-dioxabicyclo[14.1.0]heptadec-14-en-9-yl]oxy]-4-(dimethylamino)-5-hydroxy-2-methyloxan-3-yl]oxy-4-hydroxy-2,4-dimethyloxan-3-yl] 3-methylbutanoate
Computed by Lexichem TK 2.7.0 (PubChem release 2021.10.14)
InChI=1S/C42H67NO16/c1-21(2)16-32(47)57-40-25(6)53-34(20-42(40,8)50)58-37-24(5)54-41(36(49)35(37)43(9)10)59-38-27(14-15-44)17-22(3)28(46)12-13-29-30(56-29)18-23(4)52-33(48)19-31(39(38)51-11)55-26(7)45/h12-13,15,21-25,27,29-31,34-41,49-50H,14,16-20H2,1-11H3/b13-12+/t22-,23-,24-,25+,27+,29+,30+,31-,34+,35-,36-,37-,38+,39+,40+,41+,42-/m1/s1
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
FQVHOULQCKDUCY-OGHXVOSASA-N
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
C[C@@H]1C[C@@H]([C@@H]([C@H]([C@@H](CC(=O)O[C@@H](C[C@H]2[C@@H](O2)/C=C/C1=O)C)OC(=O)C)OC)O[C@H]3[C@@H]([C@H]([C@@H]([C@H](O3)C)O[C@H]4C[C@@]([C@H]([C@@H](O4)C)OC(=O)CC(C)C)(C)O)N(C)C)O)CC=O
Computed by OEChem 2.3.0 (PubChem release 2024.12.12)
C42H67NO16
Computed by PubChem 2.2 (PubChem release 2021.10.14)
- (12S,13S)-9-deoxy-12,13-epoxy-12,13-dihydro-9-oxoleucomycin v 3-acetate 4b-(3-methylbutanoate)
- carbomycin
- carbomycin A
- deltamycin A4
- Magnamycin
- NSC 51001
- NSC-51001
- CARBOMYCIN
- Carbomycin A
- Magnamycin
- Magnamycin A
- Deltamycin A4
- 4564-87-8
- AIK0XUF3AV
- M-4209
- Carbomycin acetate
- Leucomycin V, 9-deoxy-12,13-epoxy-12,13-dihydro-9-oxo-, 3-acetate 4B-(3-methylbutanoate), (12S,13S)-
- [(2S,3S,4R,6S)-6-[(2R,3S,4R,5R,6S)-6-[[(1S,3R,7R,8S,9S,10R,12R,14E,16S)-7-acetyloxy-8-methoxy-3,12-dimethyl-5,13-dioxo-10-(2-oxoethyl)-4,17-dioxabicyclo[14.1.0]heptadec-14-en-9-yl]oxy]-4-(dimethylamino)-5-hydroxy-2-methyloxan-3-yl]oxy-4-hydroxy-2,4-dimethyloxan-3-yl] 3-methylbutanoate
- Carbomicina
- Carbomycine
- Carbomycinum
- GS MAI 5201 52 3
- GS-MAI 5201-52-3
- (2S,3S,4R,6S)-6-(((2R,3S,4R,5R,6S)-6-(((1S,3R,7R,8S,9S,10R,12R,16S,E)-7-acetoxy-8-methoxy-3,12-dimethyl-5,13-dioxo-10-(2-oxoethyl)-4,17-dioxabicyclo[14.1.0]heptadec-14-en-9-yl)oxy)-4-(dimethylamino)-5-hydroxy-2-methyltetrahydro-2H-pyran-3-yl)oxy)-4-hydroxy-2,4-dimethyltetrahydro-2H-pyran-3-yl 3-methylbutanoate
- Carbomycin [INN]
- Magnamycin (VAN)
- UNII-AIK0XUF3AV
- NSC 51001
- Carbomycine [INN-French]
- Carbomycinum [INN-Latin]
- Carbomicina [INN-Spanish]
- NSC-51001
- NSC-55924
- (12S,13S)-9-Deoxy-12,13-epoxy-12,13-dihydro-9-oxoleucomycin V 3-Acetate 4B-(3-Methylbutanoate)
- AI3-50160
- CARBOMYCIN A [MI]
- M 4209
- CHEMBL1231649
- FQVHOULQCKDUCY-OGHXVOSASA-N
- BDBM667168
- HY-B1592
- Leucomycin V, 9-deoxy-12,13-epoxy-12,13-dihydro-9-oxo-, 3-acetate
- DB11383
- US20240118263, Compound Carbomycin
- CS-0013491
- (2S,3S,4R,6S)-6-{[(2R,3S,4R,5R,6S)-6-{[(1S,3R,7R,8S,9S,10R,12R,14E,16S)-7-(acetyloxy)-8-methoxy-3,12-dimethyl-5,13-dioxo-10-(2-oxoethyl)-4,17-dioxabicyclo[14.1.0]heptadec-14-en-9-yl]oxy}-4-(dimethylamino)-5-hydroxy-2-methyltetrahydro-2H-pyran-3-yl]oxy}-4-hydroxy-2,4-dimethyltetrahydro-2H-pyran-3-yl 3-methylbutanoate (non-preferred name)
- An antibiotic obtained from cultures of Streptomyces halstedii, or the same substance produced by any other means
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
842.0 g/mol
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
XLogP3-AA
Property Value
1.8
Reference
Computed by XLogP3 3.0 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Donor Count
Property Value
2
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Acceptor Count
Property Value
17
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Rotatable Bond Count
Property Value
14
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Exact Mass
Property Value
841.44598505 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Monoisotopic Mass
Property Value
841.44598505 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Topological Polar Surface Area
Property Value
215 Ų
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Heavy Atom Count
Property Value
59
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
1470
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
17
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
1
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
1
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2021.10.14)
Basic pKa
7.61
Tested as SID 104247831 in AID 781326: https://pubchem.ncbi.nlm.nih.gov/bioassay/781326#sid=104247831
Comparison of the accuracy of experimental and predicted pKa values of basic and acidic compounds. Pharm Res. 2014; 31(4):1082-95. DOI:10.1007/s11095-013-1232-z. PMID:24249037
Active Ingredients (Carbomycin) -> FDA Greenbook
NIST Number
1006697
Instrument Type
IT/ion trap
Collision Energy
0
Spectrum Type
MS2
Precursor Type
[M+H]+
Precursor m/z
842.4533
Total Peaks
18
m/z Top Peak
614
m/z 2nd Highest
615
m/z 3rd Highest
423
Thumbnail
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Same Connectivity Count
Same Parent, Connectivity Count
Similar Compounds (2D)
Similar Conformers (3D)
Same Count
Carbomycin (annotation moved to)
Animal Drugs -> FDA Approved Animal Drug Products (Green Book) -> Active Ingredients
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/
- ChemIDplusChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- DrugBankLICENSECreative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode)https://www.drugbank.ca/legal/terms_of_useCarbomycinhttps://www.drugbank.ca/drugs/DB11383
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.html
- FDA Approved Animal Drug Products (Green Book)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Japan Chemical Substance Dictionary (Nikkaji)
- Metabolomics Workbench
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuse
- NIST Mass Spectrometry Data CenterLICENSEData covered by the Standard Reference Data Act of 1968 as amended.https://www.nist.gov/srd/public-lawCarbomycin Ahttp://www.nist.gov/srd/nist1a.cfm
- Protein Data Bank in Europe (PDBe)
- RCSB Protein Data Bank (RCSB PDB)LICENSEData files contained in the PDB archive (ftp://ftp.wwpdb.org) are free of all copyright restrictions and made fully and freely available for both non-commercial and commercial use. Users of the data should attribute the original authors of that structural data.https://www.rcsb.org/pages/policies
- WikidataCarbomycinhttps://www.wikidata.org/wiki/Q15634253
- WikipediaCarbomycinhttps://en.wikipedia.org/wiki/Carbomycin
- PubChem
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.html
- EPA Substance Registry ServicesEPA SRS List Classificationhttps://sor.epa.gov/sor_internet/registry/substreg/LandingPage.do
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
CONTENTS