C.I. Pigment Green 7
- PHTHALOCYANINE GREEN
- Heliogen Green G
- Colanyl Green GG
- 31235-28-6
- BPO9294G4W
- Create:2005-08-08
- Modify:2025-02-01
- PHTHALOCYANINE GREEN
- Heliogen Green G
- Colanyl Green GG
- 31235-28-6
- BPO9294G4W
- NCI-C54637
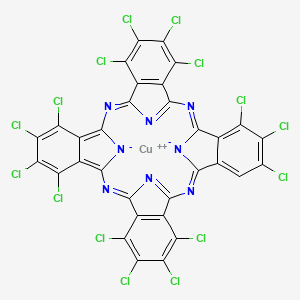
- [1,2,3,4,8,9,10,11,15,16,17,18,22,23,24-pentadecachlorophthalocyaninato(2-)]copper
- copper;5,6,7,8,15,16,17,23,24,25,26,32,33,34,35-pentadecachloro-2,11,20,29,37,38-hexaza-39,40-diazanidanonacyclo[28.6.1.13,10.112,19.121,28.04,9.013,18.022,27.031,36]tetraconta-1,3,5,7,9,11,13,15,17,19,21(38),22(27),23,25,28,30(37),31(36),32,34-nonadecaene
- Ramapo
- Fastolux Green
- SolFast Green
- Synthaline Green
- Cyanine Green T
- Fenalac Green G
- Versal Green G
- Fastogen Green B
- Heliogen Green A
- (1,2,3,4,8,9,10,11,15,16,17,18,22,23,24-Pentadecachlorophthalocyaninato(2-))copper
- Cyanine Green GP
- Cyanine Green NB
- Monarch Green WD
- Vynamon Green BE
- Calcotone Green G
- Ceres Green 3B
- Chromatex Green G
- Monastral Green B
- Monastral Green G
- Heliogen Green GA
- Heliogen Green GN
- Heliogen Green GV
- Microlit Green GK
- Pv-Fast Green G
- Vynamon Green BES
- Vynamon Green GNA
- Hostaperm Green GG
- Monastral Green GH
- Monastral Green GN
- Cyanine Green Toner
- Heliogen Green GNA
- Heliogen Green GTA
- Heliogen Green GWS
- Irgalite Green GLN
- Sanyo Cyanine Green
- Cromophtal Green GF
- Opaline Green G 1
- Monastral Green GFN
- Cromophthal Green GF
- Siegle Fast Green G
- Graphtol Green 2GLS
- Fenalac Green G Disp
- SUNFAST GREEN
- Thalo Green No. 1
- Microlith Green G-FP
- Phthalocyanine Green V
- Segnale Light Green G
- Granada Green Lake GL
- Lutetia Fast Emerald J
- Monastral Fast Green G
- Duratint Green 1001
- Fastogen Green 5005
- Heliogen Green 8680
- Heliogen Green 8730
- Phthalocyanine Green LX
- Vulcal Fast Green F2G
- Vulcol Fast Green F2G
- Accosperse Cyan Green G
- Daltolite Fast Green GN
- Heliogen Green 8681K
- Heliogen Green 8682T
- Monastral Fast Green GD
- Monastral Fast Green GF
- Monastral Fast Green GN
- Monastral Fast Green GV
- Monastral Fast Green GX
- Vulcanosine Fast Green G
- SolFast Green 63102
- Monastral Fast Green GNA
- Monastral Fast Green GTP
- Monastral Fast Green GWD
- Monastral Fast Green GXB
- Monastral Fast Green GYH
- Monolite Fast Green GVSA
- Dainichi Cyanine Green FG
- Sherwood Green A 4436
- Copper Phthalocyanine Green
- Monastral Fast Green BGNA
- Monastral Fast Green GFNP
- Monastral Fast Green LGNA
- Copper, (pentadecachlorophthalocyaninato(2-))-
- Klondike Yellow X-2261
- Termosolido Green FG Supra
- Dainichi Cyanine Green FGH
- Monastral Fast Green 2GWD
- Cyan Green 15-3100
- UNII-BPO9294G4W
- Non-flocculating Green G 25
- Phthalocyanine Green WDG 47
- Brilliant Green Phthalocyanine
- Phthalocyanine Brilliant Green
- CCRIS 4702
- Sanyo Phthalocyanine Green F6G
- Permanent Green Toner GT-376
- HSDB 4198
- Phthalocyanine Green VFT 1080
- Irgalite Fast Brilliant Green GL
- GUYIZQZWDFCUTA-UHFFFAOYSA-N
- Irgalite Fast Brilliant Green 3GL
- Sanyo Phthalocyanine Green FB Pure
- EINECS 215-524-7
- EINECS 250-523-5
- Resinated Phthalocyanine Green G-5025
- CI 72460
- CI 74260
- C.I. 72460
- EC 215-524-7
- Copper, (1,2,3,4,8,9,10,11,15,16,17,18,22,23,24-pentadecachloro-29H,31H-phthalocyaninato(2-)-kappaN29,kappaN30,kappaN31,kappaN32)-, (SP-4-2)-
- Copper, [1,2,3,4,8,9,10,11,15,16,17,18,22,23,24-pentadecachloro-29H,31H-phthalocyaninato(2-)-.kappa.N29,.kappa.N30,.kappa.N31,.kappa.N32]-, (SP-4-2)-
Pulp and Paper Processing [Category: Industry]
Painting (Pigments, Binders, and Biocides) [Category: Paint]
Leather Tanning and Processing [Category: Industry]
Textiles (Printing, Dyeing, or Finishing) [Category: Industry]
 Green circle - The chemical has been verified to be of low concern
Green circle - The chemical has been verified to be of low concern- Pigment
- Dyes
- Pigments
- Paint additives and coating additives not described by other categories
- Adhesion/cohesion promoter
- Pigment
- Not Known or Reasonably Ascertainable
- Dyes
- Pigments
- Paint additives and coating additives not described by other categories
- Agricultural chemicals (non-pesticidal)
Information on 31 consumer products that contain Phthalocyanine Green in the following categories is provided:
• Home Maintenance
• Inside the Home
2019: 1,000,000 - <10,000,000 lb
2018: 1,000,000 - <10,000,000 lb
2017: 1,000,000 - <10,000,000 lb
2016: 1,000,000 - <10,000,000 lb
- Wholesale and Retail Trade
- Miscellaneous Manufacturing
- Paint and Coating Manufacturing
- Synthetic Dye and Pigment Manufacturing
- Printing Ink Manufacturing
- Plastics Product Manufacturing
- Not Known or Reasonably Ascertainable
- Plastics Material and Resin Manufacturing
- Construction
- Custom Compounding of Purchased Resins
- All Other Chemical Product and Preparation Manufacturing
- Printing and Related Support Activities
Chemical: C.I. Pigment Green 7

EYES: First check the victim for contact lenses and remove if present. Flush victim's eyes with water or normal saline solution for 20 to 30 minutes while simultaneously calling a hospital or poison control center. Do not put any ointments, oils, or medication in the victim's eyes without specific instructions from a physician. IMMEDIATELY transport the victim after flushing eyes to a hospital even if no symptoms (such as redness or irritation) develop.
SKIN: IMMEDIATELY flood affected skin with water while removing and isolating all contaminated clothing. Gently wash all affected skin areas thoroughly with soap and water. If symptoms such as redness or irritation develop, IMMEDIATELY call a physician and be prepared to transport the victim to a hospital for treatment.
INHALATION: IMMEDIATELY leave the contaminated area; take deep breaths of fresh air. If symptoms (such as wheezing, coughing, shortness of breath, or burning in the mouth, throat, or chest) develop, call a physician and be prepared to transport the victim to a hospital. Provide proper respiratory protection to rescuers entering an unknown atmosphere. Whenever possible, Self-Contained Breathing Apparatus (SCBA) should be used; if not available, use a level of protection greater than or equal to that advised under Protective Clothing.
INGESTION: DO NOT INDUCE VOMITING. If the victim is conscious and not convulsing, give 1 or 2 glasses of water to dilute the chemical and IMMEDIATELY call a hospital or poison control center. Be prepared to transport the victim to a hospital if advised by a physician. If the victim is convulsing or unconscious, do not give anything by mouth, ensure that the victim's airway is open and lay the victim on his/her side with the head lower than the body. DO NOT INDUCE VOMITING. IMMEDIATELY transport the victim to a hospital. (NTP, 1992)
SMALL SPILLS AND LEAKAGE: If you spill this chemical, you should dampen the solid spill material with 5% acetic acid, then transfer the dampened material to a suitable container. Use absorbent paper dampened with 5% acetic acid to pick up any remaining material. Your contaminated clothing and the absorbent paper should be sealed in a vapor-tight plastic bag for eventual disposal. Wash all contaminated surfaces with 5% acetic acid followed by washing with a soap and water solution. Do not reenter the contaminated area until the Safety Officer (or other responsible person) has verified that the area has been properly cleaned.
STORAGE PRECAUTIONS: You should store this material in a refrigerator. (NTP, 1992)
Amines, Phosphines, and Pyridines
Non-Redox-Active Inorganic Compounds
Aryl Halides
- Australian Industrial Chemicals Introduction Scheme (AICIS)C.I. Pigment Green 7https://services.industrialchemicals.gov.au/search-assessments/C.I. Pigment Green 7https://services.industrialchemicals.gov.au/search-inventory/
- CAMEO ChemicalsLICENSECAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data.https://cameochemicals.noaa.gov/help/reference/terms_and_conditions.htm?d_f=falseC.I. PIGMENT GREEN 7https://cameochemicals.noaa.gov/chemical/20917
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/(SP-4-2)-[1,2,3,4,8,9,10,11,15,16,17,18,22,23,24-Pentadecachloro-29H,31H-phthalocyaninato(2-)-κN29,κN30,κN31,κN32]copperhttps://commonchemistry.cas.org/detail?cas_rn=31235-28-6Pigment Green 7https://commonchemistry.cas.org/detail?cas_rn=1328-53-6
- ChemIDplusPhthalocyanine Greenhttps://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0001328536Copper, (pentadecachlorophthalocyaninato(2-))-https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0031235286ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA Chemical Data Reporting (CDR)LICENSEThe U.S. Government retains a nonexclusive, royalty-free license to publish or reproduce these documents, or allow others to do so, for U.S. Government purposes. These documents may be freely distributed and used for non-commercial, scientific and educational purposes.https://www.epa.gov/web-policies-and-procedures/epa-disclaimers#copyrightC.I. Pigment Green 7https://www.epa.gov/chemical-data-reporting
- EPA Chemicals under the TSCAC.I. Pigment Green 7https://www.epa.gov/chemicals-under-tscaEPA TSCA Classificationhttps://www.epa.gov/tsca-inventory
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-notice[1,2,3,4,8,9,10,11,15,16,17,18,22,23,24-pentadecachlorophthalocyaninato(2-)]copperhttps://echa.europa.eu/substance-information/-/substanceinfo/100.045.915
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Hazardous Substances Data Bank (HSDB)PHTHALOCYANINE GREENhttps://pubchem.ncbi.nlm.nih.gov/source/hsdb/4198
- New Zealand Environmental Protection Authority (EPA)LICENSEThis work is licensed under the Creative Commons Attribution-ShareAlike 4.0 International licence.https://www.epa.govt.nz/about-this-site/general-copyright-statement/
- Haz-Map, Information on Hazardous Chemicals and Occupational DiseasesLICENSECopyright (c) 2022 Haz-Map(R). All rights reserved. Unless otherwise indicated, all materials from Haz-Map are copyrighted by Haz-Map(R). No part of these materials, either text or image may be used for any purpose other than for personal use. Therefore, reproduction, modification, storage in a retrieval system or retransmission, in any form or by any means, electronic, mechanical or otherwise, for reasons other than personal use, is strictly prohibited without prior written permission.https://haz-map.com/AboutPhthalocyanine Greenhttps://haz-map.com/Agents/6707
- Consumer Product Information Database (CPID)LICENSECopyright (c) 2024 DeLima Associates. All rights reserved. Unless otherwise indicated, all materials from CPID are copyrighted by DeLima Associates. No part of these materials, either text or image may be used for any purpose other than for personal use. Therefore, reproduction, modification, storage in a retrieval system or retransmission, in any form or by any means, electronic, mechanical or otherwise, for reasons other than personal use, is strictly prohibited without prior written permission.https://www.whatsinproducts.com/contents/view/1/6Phthalocyanine Greenhttps://www.whatsinproducts.com/chemicals/view/1/4290/001328-53-6Consumer Products Category Classificationhttps://www.whatsinproducts.com/
- EPA Safer ChoiceC.I. Pigment Green 7https://www.epa.gov/saferchoice/safer-ingredients
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/NORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- FDA Regulatory Status of Color Additives
- NITE-CMCPhthalocyanine Green - FY2016 (New/original classication)https://www.chem-info.nite.go.jp/chem/english/ghs/16-mhlw-0044e.html
- NLM RxNorm TerminologyLICENSEThe RxNorm Terminology is created by the National Library of Medicine (NLM) and is in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from NLM. Credit to the U.S. National Library of Medicine as the source is appreciated but not required. The full RxNorm dataset requires a free license.https://www.nlm.nih.gov/research/umls/rxnorm/docs/termsofservice.htmlpigment green 7https://rxnav.nlm.nih.gov/id/rxnorm/1918055
- WikidataCopper(2+) 1,2,3,4,9,10,11,15,16,17,18,22,23,24,25-pentadecachlorophthalocyanine-6,29-diidehttps://www.wikidata.org/wiki/Q82932110
- PubChem
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlphthalocyanine greenhttps://www.ncbi.nlm.nih.gov/mesh/67505810
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- EPA Substance Registry ServicesEPA SRS List Classificationhttps://sor.epa.gov/sor_internet/registry/substreg/LandingPage.do
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/

 CID 135993870
CID 135993870 CID 23978 (Copper)
CID 23978 (Copper) CID 135566093
CID 135566093