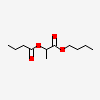
Butyl Butyryllactate
- Butyl butyryllactate
- 7492-70-8
- Butyl butyryl lactate
- Butyl butyrolactate
- Butyl O-butyryllactate
- Create:2005-03-26
- Modify:2025-01-18
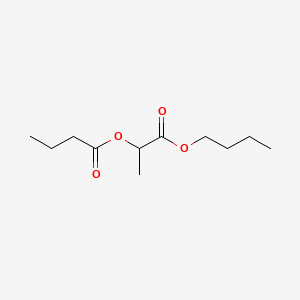
- Butyl butyryllactate
- 7492-70-8
- Butyl butyryl lactate
- Butyl butyrolactate
- Butyl O-butyryllactate
- Butanoic acid, 2-butoxy-1-methyl-2-oxoethyl ester
- 1-Butoxy-1-oxopropan-2-yl butyrate
- 1-butoxy-1-oxopropan-2-yl butanoate
- BUTYRIC ACID, ester with BUTYL LACTATE
- (1-butoxy-1-oxopropan-2-yl) butanoate
- FEMA No. 2190
- Lactic acid, butyl ester, butyrate
- Butyl butyryl lactate (natural)
- 2-Butoxy-1-methyl-2-oxoethyl butanoate
- UNII-OCR0ONT89C
- OCR0ONT89C
- EINECS 231-326-3
- NSC 166507
- BRN 1709603
- Butyl 2-(Butyryloxy)propionate
- DTXSID3044831
- O-Butyryllactic Acid Butyl Ester
- BUTYRYLLACTIC ACID
- n-Butyl n-butyryl lactate
- NSC-166507
- DTXCID1024831
- FEMA 2190
- CHEBI:168668
- BUTYL BUTYRYLLACTATE [FCC]
- 3-03-00-00481 (Beilstein Handbook Reference)
- BUTYL BUTYRYL LACTATE [FHFI]
- 2-(Butyryloxy)propionic Acid Butyl Ester
- BUTYLBUTYRYL LACTATE
- MFCD00027213
- Butanoic acid 2-butoxy-1-methyl-2-oxoethyl ester
- SCHEMBL891453
- Tox21_301784
- butyric acid ester with butyl lactate
- LMFA07010792
- NSC166507
- AKOS016009696
- 2-Butoxy-1-methyl-2-oxoethyl butyrate
- NCGC00256245-01
- BS-18050
- Butyl butyryllactate, >=98%, FCC, FG
- 2-Butoxy-1-methyl-2-oxoethyl butyrate #
- CAS-7492-70-8
- B4344
- NS00012029
- 2-Butoxy-1-methyl-2-oxoethyl butanoate, 9CI
- Butyl butyryllactate, natural (US), >=98%, FG
- W-104404
- Q27285582
71.0 99.99
41.0 78.20
115.0 13.50
57.0 6.30
29.0 5.40
71.0 99.99
43.0 33
27.0 9.30
57.0 9
29.0 8
71 999
41 782
115 135
57 63
29 54
71 999
43 330
27 93
57 90
29 80
- Extracellular
- Membrane
Information on 12 consumer products that contain Butyl Butyrolactate in the following categories is provided:
• Inside the Home
Not Classified
Reported as not meeting GHS hazard criteria by 1576 of 1618 companies (only 2.6% companies provided GHS information). For more detailed information, please visit ECHA C&L website.
Aggregated GHS information provided per 1618 reports by companies from 5 notifications to the ECHA C&L Inventory.
Reported as not meeting GHS hazard criteria per 1576 of 1618 reports by companies. For more detailed information, please visit ECHA C&L website.
There are 4 notifications provided by 42 of 1618 reports by companies with hazard statement code(s).
Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown.
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=NORZZKKLCYMBBF-UHFFFAOYSA-N
- Australian Industrial Chemicals Introduction Scheme (AICIS)Butanoic acid, 2-butoxy-1-methyl-2-oxoethyl esterhttps://services.industrialchemicals.gov.au/search-inventory/
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/Butyl butyryllactatehttps://commonchemistry.cas.org/detail?cas_rn=7492-70-8
- ChemIDplusButyl butyryllactatehttps://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0007492708ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- DTP/NCILICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuse
- EPA Chemical Data Reporting (CDR)LICENSEThe U.S. Government retains a nonexclusive, royalty-free license to publish or reproduce these documents, or allow others to do so, for U.S. Government purposes. These documents may be freely distributed and used for non-commercial, scientific and educational purposes.https://www.epa.gov/web-policies-and-procedures/epa-disclaimers#copyrightButanoic acid, 2-butoxy-1-methyl-2-oxoethyl esterhttps://www.epa.gov/chemical-data-reporting
- EPA Chemicals under the TSCAButanoic acid, 2-butoxy-1-methyl-2-oxoethyl esterhttps://www.epa.gov/chemicals-under-tscaEPA TSCA Classificationhttps://www.epa.gov/tsca-inventory
- EPA DSSTox1-Butoxy-1-oxopropan-2-yl butanoatehttps://comptox.epa.gov/dashboard/DTXSID3044831CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-noticeButyl O-butyryllactatehttps://chem.echa.europa.eu/100.028.479Butyl O-butyryllactate (EC: 231-326-3)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/8067
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingBUTYL BUTYRYLLACTATEhttps://gsrs.ncats.nih.gov/ginas/app/beta/substances/OCR0ONT89C
- Human Metabolome Database (HMDB)LICENSEHMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications.http://www.hmdb.ca/citingButyl butyryllactatehttp://www.hmdb.ca/metabolites/HMDB0037137HMDB0037137_cms_28947https://hmdb.ca/metabolites/HMDB0037137#spectra
- International Fragrance Association (IFRA)LICENSE(c) The International Fragrance Association, 2007-2021. All rights reserved.https://ifrafragrance.org/links/copyrightButyl butyryllactatehttps://ifrafragrance.org/priorities/ingredients/ifra-transparency-list
- New Zealand Environmental Protection Authority (EPA)LICENSEThis work is licensed under the Creative Commons Attribution-ShareAlike 4.0 International licence.https://www.epa.govt.nz/about-this-site/general-copyright-statement/
- ChEBIButyl butyryllactatehttps://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:168668
- LOTUS - the natural products occurrence databaseLICENSEThe code for LOTUS is released under the GNU General Public License v3.0.https://lotus.nprod.net/Butyl butyryllactatehttps://www.wikidata.org/wiki/Q27285582LOTUS Treehttps://lotus.naturalproducts.net/
- Consumer Product Information Database (CPID)LICENSECopyright (c) 2024 DeLima Associates. All rights reserved. Unless otherwise indicated, all materials from CPID are copyrighted by DeLima Associates. No part of these materials, either text or image may be used for any purpose other than for personal use. Therefore, reproduction, modification, storage in a retrieval system or retransmission, in any form or by any means, electronic, mechanical or otherwise, for reasons other than personal use, is strictly prohibited without prior written permission.https://www.whatsinproducts.com/contents/view/1/6Butyl Butyrolactatehttps://www.whatsinproducts.com/chemicals/view/1/5371/007492-70-8Consumer Products Category Classificationhttps://www.whatsinproducts.com/
- EPA Chemical and Products Database (CPDat)1-Butoxy-1-oxopropan-2-yl butanoatehttps://comptox.epa.gov/dashboard/DTXSID3044831#exposureEPA CPDat Classificationhttps://www.epa.gov/chemical-research/chemical-and-products-database-cpdat
- EU Food Improvement AgentsButyl-O-butyryllactatehttps://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32012R0872
- Joint FAO/WHO Expert Committee on Food Additives (JECFA)LICENSEPermission from WHO is not required for the use of WHO materials issued under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Intergovernmental Organization (CC BY-NC-SA 3.0 IGO) licence.https://www.who.int/about/policies/publishing/copyrightBUTYL BUTYROLACTATEhttps://apps.who.int/food-additives-contaminants-jecfa-database/Home/Chemical/3853
- FDA Substances Added to FoodLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Flavor and Extract Manufacturers Association (FEMA)BUTYL BUTYRYLLACTATEhttps://www.femaflavor.org/flavor-library/butyl-butyryllactate
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- NIST Mass Spectrometry Data CenterLICENSEData covered by the Standard Reference Data Act of 1968 as amended.https://www.nist.gov/srd/public-lawButanoic acid, 2-butoxy-1-methyl-2-oxoethyl esterhttp://www.nist.gov/srd/nist1a.cfm
- SpectraBaseButyl butyryl lactatehttps://spectrabase.com/spectrum/1x3eHIvLP5bButyryllactate <butyl->https://spectrabase.com/spectrum/F871dQsVUnnBUTYL BUTYRYL LACTATEhttps://spectrabase.com/spectrum/9kgFGzYJd2cButyl butyryllactatehttps://spectrabase.com/spectrum/LfXHxuPMcg9LACTIC ACID, BUTYL ESTER, BUTYRATEhttps://spectrabase.com/spectrum/B2QX08cB36GLactic acid, butyl ester butyratehttps://spectrabase.com/spectrum/4DJLFENBFq0LACTIC ACID, BUTYL ESTER, BUTYRATEhttps://spectrabase.com/spectrum/8J0rLregp4iButyl butyryllactatehttps://spectrabase.com/spectrum/GZiAKZj3713Butyl butyryllactatehttps://spectrabase.com/spectrum/GtB83m2HMS9
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/NORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- Japan Chemical Substance Dictionary (Nikkaji)
- LIPID MAPSLipid Classificationhttps://www.lipidmaps.org/
- MassBank EuropeBUTYL BUTYRYLLACTATEhttps://massbank.eu/MassBank/Result.jsp?inchikey=NORZZKKLCYMBBF-UHFFFAOYSA-N
- Metabolomics WorkbenchButyl butyryllactatehttps://www.metabolomicsworkbench.org/data/StructureData.php?RegNo=47628
- Wikidatabutyl butyryllactatehttps://www.wikidata.org/wiki/Q27285582
- PubChem
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- EPA Substance Registry ServicesEPA SRS List Classificationhttps://sor.epa.gov/sor_internet/registry/substreg/LandingPage.do
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 403656011https://pubchem.ncbi.nlm.nih.gov/substance/403656011