Atosiban Acetate
PubChem CID
87665603
Molecular Formula
Synonyms
- Atosiban acetate
- 914453-95-5
- UNII-0P5DNO7CEF
- 0P5DNO7CEF
- ATOSIBAN ACETATE [WHO-DD]
Molecular Weight
1054.2 g/mol
Computed by PubChem 2.2 (PubChem release 2024.11.20)
Parent Compound
Component Compounds
Dates
- Create:2015-02-12
- Modify:2025-01-18
Description
ATOSIBAN ACETATE is a Protein drug with a maximum clinical trial phase of IV that was first approved in 2000 and is indicated for premature birth.
Chemical Structure Depiction
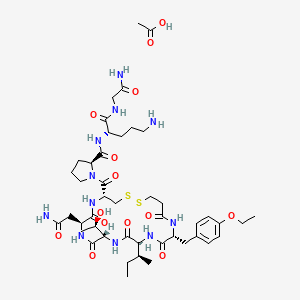
Conformer generation is disallowed since too many atoms, too flexible, mixture or salt
SVG Image

IUPAC Condensed
deamino-Cys(1)-D-Tyr(Et)-Ile-Thr-Asn-Cys(1)-Pro-Orn-Gly-NH2.CH3CO2H
Sequence
CXITNCPXG
IUPAC
deamino-cysteinyl-O4-ethyl-D-tyrosyl-L-isoleucyl-L-threonyl-L-asparagyl-L-cysteinyl-L-prolyl-L-ornithyl-glycinamide (1->6)-disulfide acetic acid
acetic acid;(2S)-N-[(2S)-5-amino-1-[(2-amino-2-oxoethyl)amino]-1-oxopentan-2-yl]-1-[(4R,7S,10S,13S,16R)-7-(2-amino-2-oxoethyl)-13-[(2S)-butan-2-yl]-16-[(4-ethoxyphenyl)methyl]-10-[(1R)-1-hydroxyethyl]-6,9,12,15,18-pentaoxo-1,2-dithia-5,8,11,14,17-pentazacycloicosane-4-carbonyl]pyrrolidine-2-carboxamide
Computed by Lexichem TK 2.7.0 (PubChem release 2024.11.20)
InChI=1S/C43H67N11O12S2.C2H4O2/c1-5-23(3)35-41(63)53-36(24(4)55)42(64)50-29(20-32(45)56)38(60)51-30(43(65)54-17-8-10-31(54)40(62)49-27(9-7-16-44)37(59)47-21-33(46)57)22-68-67-18-15-34(58)48-28(39(61)52-35)19-25-11-13-26(14-12-25)66-6-2;1-2(3)4/h11-14,23-24,27-31,35-36,55H,5-10,15-22,44H2,1-4H3,(H2,45,56)(H2,46,57)(H,47,59)(H,48,58)(H,49,62)(H,50,64)(H,51,60)(H,52,61)(H,53,63);1H3,(H,3,4)/t23-,24+,27-,28+,29-,30-,31-,35-,36-;/m0./s1
Computed by InChI 1.07.0 (PubChem release 2024.11.20)
SVDWBHHCPXTODI-QIWYXCRTSA-N
Computed by InChI 1.07.0 (PubChem release 2024.11.20)
CC[C@H](C)[C@H]1C(=O)N[C@H](C(=O)N[C@H](C(=O)N[C@@H](CSSCCC(=O)N[C@@H](C(=O)N1)CC2=CC=C(C=C2)OCC)C(=O)N3CCC[C@H]3C(=O)N[C@@H](CCCN)C(=O)NCC(=O)N)CC(=O)N)[C@@H](C)O.CC(=O)O
Computed by OEChem 2.3.0 (PubChem release 2024.12.12)
C45H71N11O14S2
Computed by PubChem 2.2 (PubChem release 2024.11.20)
914453-95-5
- Atosiban acetate
- 914453-95-5
- UNII-0P5DNO7CEF
- 0P5DNO7CEF
- ATOSIBAN ACETATE [WHO-DD]
- ATOSIBAN ACETATE [EMA EPAR]
- acetic acid;(2S)-N-[(2S)-5-amino-1-[(2-amino-2-oxoethyl)amino]-1-oxopentan-2-yl]-1-[(4R,7S,10S,13S,16R)-7-(2-amino-2-oxoethyl)-13-[(2S)-butan-2-yl]-16-[(4-ethoxyphenyl)methyl]-10-[(1R)-1-hydroxyethyl]-6,9,12,15,18-pentaoxo-1,2-dithia-5,8,11,14,17-pentazacycloicosane-4-carbonyl]pyrrolidine-2-carboxamide
- GLYCINAMIDE, O-ETHYL-N-(3-MERCAPTO-1-OXOPROPYL)-D-TYROSYL-L-ISOLEUCYL-L-THREONYL-L-ASPARAGINYL-L-CYSTEINYL-L-PROLYL-L-ORNITHYL-, CYCLIC (1->5)-DISULFIDE, ACETATE (1:1)
- GLYCINAMIDE, O-ETHYL-N-(3-MERCAPTO-1-OXOPROPYL)-D-TYROSYL-L-ISOLEUCYL-L-THREONYL-L-ASPARAGINYL-L-CYSTEINYL-L-PROLYL-L-ORNITHYL-, CYCLIC (1->5)-DISULFIDE, MONOACETATE (SALT)
- GLYCINAMIDE, O-ETHYL-N-(3-MERCAPTO-1-OXOPROPYL)-D-TYROSYL-L-ISOLEUCYL-L-THREONYL-L-ASPARAGINYL-L-CYSTEINYL-L-PROLYL-L-ORNITHYL-, CYCLIC (1->5)-DISULPHIDE, ACETATE (1:1)
- GLYCINAMIDE, O-ETHYL-N-(3-MERCAPTO-1-OXOPROPYL)-D-TYROSYL-L-ISOLEUCYL-L-THREONYL-L-ASPARAGINYL-L-CYSTEINYL-L-PROLYL-L-ORNITHYL-, CYCLIC (1->5)-DISULPHIDE, MONOACETATE (SALT)
- OXYTOCIN, 1-(3-MERCAPTOPROPANOIC ACID)-2-(O-ETHYL-D-TYROSINE)-4-L-THREONINE-8-L-ORNITHINE-, ACETATE
- DTXSID60238569
- Atosiban (as acetate)
- SCHEMBL4410202
- CHEMBL5315050
- DTXCID60161060
- EX-A7437
- C45H71N11O14S2
- MFCD08692014
- AKOS030485985
- DA-50781
- G13510
- Q27237053
- (Deamino-Cys1,D-Tyr(Et)2,Thr4,Orn8)-Oxytocin Acetate
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
1054.2 g/mol
Reference
Computed by PubChem 2.2 (PubChem release 2024.11.20)
Property Name
Hydrogen Bond Donor Count
Property Value
12
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2024.11.20)
Property Name
Hydrogen Bond Acceptor Count
Property Value
17
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2024.11.20)
Property Name
Rotatable Bond Count
Property Value
18
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2024.11.20)
Property Name
Exact Mass
Property Value
1053.46233833 Da
Reference
Computed by PubChem 2.2 (PubChem release 2024.11.20)
Property Name
Monoisotopic Mass
Property Value
1053.46233833 Da
Reference
Computed by PubChem 2.2 (PubChem release 2024.11.20)
Property Name
Topological Polar Surface Area
Property Value
454 Ų
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2024.11.20)
Property Name
Heavy Atom Count
Property Value
72
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
1810
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2024.11.20)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
9
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
2
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2021.10.14)
Human drugs -> Other gynecologicals -> Human pharmacotherapeutic group -> EMA Drug Category
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Same Connectivity Count
Same Parent, Connectivity Count
Same Parent, Exact Count
Mixtures, Components, and Neutralized Forms Count
Similar Compounds (2D)
Similar Conformers (3D)
Same Count
Tractotile is indicated to delay imminent pre-term birth in pregnant adult women with: regular uterine contractions of at least 30 seconds duration at a rate of ⥠4 per 30 minutes; a cervical dilation of 1 to 3 cm (0-3 for nulliparas) and effacement of ⥠50%; a gestational age from 24 until 33 completed weeks; a normal foetal heart rate.
Atosiban is indicated to delay imminent pre-term birth in pregnant adult women with: regular uterine contractions of at least 30 seconds' duration at a rate of ⥠4 per 30 minutes; a cervical dilation of 1 to 3 cm (0-3 for nulliparas) and effacement of ⥠50%; a gestational age from 24 until 33 completed weeks; a normal foetal heart rate.
Medicine
Category
Human drugs
Therapeutic area
Premature Birth
Active Substance
atosiban (as acetate)
INN/Common name
atosiban
Pharmacotherapeutic Classes
Other gynecologicals
Status
This medicine is authorized for use in the European Union
Company
Ferring Pharmaceuticals A/S
Market Date
2000-01-20
Medicine
Category
Human drugs
Therapeutic area
Premature Birth
Active Substance
atosiban (as acetate)
INN/Common name
atosiban
Pharmacotherapeutic Classes
Other gynecologicals
Status
This medicine is authorized for use in the European Union
Company
Sun Pharmaceutical Industries Europe B.V.
Market Date
2013-07-31
G02CX01
Human drugs -> Other gynecologicals -> Human pharmacotherapeutic group -> EMA Drug Category
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.html
- ChemIDplusChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingATOSIBAN ACETATEhttps://gsrs.ncats.nih.gov/ginas/app/beta/substances/0P5DNO7CEF
- European Medicines Agency (EMA)LICENSEInformation on the European Medicines Agency's (EMA) website is subject to a disclaimer and copyright and limited reproduction notices.https://www.ema.europa.eu/en/about-us/legal-noticeTractocile (EMEA/H/C/000253)https://www.ema.europa.eu/en/medicines/human/EPAR/tractocileAtosiban SUN (EMEA/H/C/002329)https://www.ema.europa.eu/en/medicines/human/EPAR/atosiban-sun
- Open TargetsLICENSEDatasets generated by the Open Targets Platform are freely available for download.https://platform-docs.opentargets.org/licenceATOSIBAN ACETATEhttps://platform.opentargets.org/drug/CHEMBL5315050
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- Wikidataatosiban acetatehttps://www.wikidata.org/wiki/Q27237053
- PubChem
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- NCBI
CONTENTS
 CID 5311010 (Atosiban)
CID 5311010 (Atosiban) CID 176 (Acetic Acid)
CID 176 (Acetic Acid)