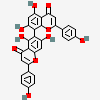
Agathisflavone
PubChem CID
5281599
Molecular Formula
Synonyms
- Agathisflavone
- 28441-98-7
- 6,8''-Biapigenin
- 7''-methyl-agathisflavone
- CHEBI:2512
Molecular Weight
538.5 g/mol
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Dates
- Create:2004-09-16
- Modify:2025-01-18
Description
Agathisflavone is a biflavonoid that is obtained by oxidative coupling of two molecules of apigenin resulting in a bond between positions C-6 and C-8 of the two chromene rings. It has a role as an antineoplastic agent, an antiviral agent, a hepatoprotective agent and a metabolite. It is a biflavonoid, a hydroxyflavone and a biaryl.
Agathisflavone has been reported in Rhus punjabensis, Toxicodendron succedaneum, and other organisms with data available.
Chemical Structure Depiction

8-[5,7-dihydroxy-2-(4-hydroxyphenyl)-4-oxochromen-6-yl]-5,7-dihydroxy-2-(4-hydroxyphenyl)chromen-4-one
Computed by Lexichem TK 2.7.0 (PubChem release 2021.10.14)
InChI=1S/C30H18O10/c31-15-5-1-13(2-6-15)22-11-20(36)26-24(39-22)12-21(37)27(29(26)38)28-18(34)9-17(33)25-19(35)10-23(40-30(25)28)14-3-7-16(32)8-4-14/h1-12,31-34,37-38H
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
BACLASYRJRZXMY-UHFFFAOYSA-N
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
C1=CC(=CC=C1C2=CC(=O)C3=C(O2)C=C(C(=C3O)C4=C(C=C(C5=C4OC(=CC5=O)C6=CC=C(C=C6)O)O)O)O)O
Computed by OEChem 2.3.0 (PubChem release 2024.12.12)
C30H18O10
Computed by PubChem 2.2 (PubChem release 2021.10.14)
- 7''-methyl-agathisflavone
- agathisflavone
- Agathisflavone
- 28441-98-7
- 6,8''-Biapigenin
- 7''-methyl-agathisflavone
- CHEBI:2512
- 8-[5,7-dihydroxy-2-(4-hydroxyphenyl)-4-oxochromen-6-yl]-5,7-dihydroxy-2-(4-hydroxyphenyl)chromen-4-one
- DTXSID30182641
- (6,8'-Bi-4H-1-benzopyran)-4,4'-dione, 5,5',7,7'-tetrahydroxy-2,2'-bis(4-hydroxyphenyl)-
- 5,5',7,7'-tetrahydroxy-2,2'-bis(4-hydroxyphenyl)-(6,8'-bi-4H-1-benzopyran)-4,4'-dione
- 5,5',7,7'-tetrahydroxy-2,2'-bis(4-hydroxyphenyl)-6,8'-bichromene-4,4'-dione
- 8-(5,7-dihydroxy-2-(4-hydroxyphenyl)-4-oxochromen-6-yl)-5,7-dihydroxy-2-(4-hydroxyphenyl)chromen-4-one
- CHEMBL65320
- SCHEMBL616142
- DTXCID70105132
- BDBM50478418
- LMPK12040008
- AKOS040745510
- HY-118383
- CS-0065801
- C10017
- Q27105695
- 5,5',7,7'-Tetrahydroxy-2,2'-bis(4-hydroxyphenyl)-4H,4'H-[6,8'-bichromene]-4,4'-dione
- 8-[5,7-dihydroxy-2-(4-hydroxyphenyl)-4-oxo-chromen-6-yl]-5,7-dihydroxy-2-(4-hydroxyphenyl)chromen-4-one
- NCGC00384551-01!8-[5,7-dihydroxy-2-(4-hydroxyphenyl)-4-oxochromen-6-yl]-5,7-dihydroxy-2-(4-hydroxyphenyl)chromen-4-one
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
538.5 g/mol
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
XLogP3-AA
Property Value
5
Reference
Computed by XLogP3 3.0 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Donor Count
Property Value
6
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Acceptor Count
Property Value
10
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Rotatable Bond Count
Property Value
3
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Exact Mass
Property Value
538.08999677 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Monoisotopic Mass
Property Value
538.08999677 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Topological Polar Surface Area
Property Value
174 Ų
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Heavy Atom Count
Property Value
40
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
1040
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
1
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2021.10.14)
Polyketides [PK] -> Flavonoids [PK12] -> Biflavonoids and polyflavonoids [PK1204]
MoNA ID
MS Category
Experimental
MS Type
LC-MS
MS Level
MS2
Precursor Type
[M-H]-
Precursor m/z
537.083
Instrument
Maxis II HD Q-TOF Bruker
Ionization Mode
negative
Top 5 Peaks
537.080872 100
375.055084 44.49
443.042755 21.04
417.054443 10.79
307.069122 8.58
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Mixtures, Components, and Neutralized Forms Count
Similar Compounds (2D)
Similar Conformers (3D)
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=BACLASYRJRZXMY-UHFFFAOYSA-N
The LOTUS Initiative for Open Natural Products Research: frozen dataset union wikidata (with metadata) | DOI:10.5281/zenodo.5794106
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/Agathisflavonehttps://commonchemistry.cas.org/detail?cas_rn=28441-98-7
- ChemIDplusChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA DSSToxAgathisflavonehttps://comptox.epa.gov/dashboard/DTXSID30182641CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- ChEBI
- LOTUS - the natural products occurrence databaseLICENSEThe code for LOTUS is released under the GNU General Public License v3.0.https://lotus.nprod.net/Agathisflavonehttps://www.wikidata.org/wiki/Q27105695LOTUS Treehttps://lotus.naturalproducts.net/
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- Comparative Toxicogenomics Database (CTD)LICENSEIt is to be used only for research and educational purposes. Any reproduction or use for commercial purpose is prohibited without the prior express written permission of NC State University.http://ctdbase.org/about/legal.jspagathisflavonehttps://ctdbase.org/detail.go?type=chem&acc=C079168
- Japan Chemical Substance Dictionary (Nikkaji)
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.htmlPhytochemical compoundshttp://www.genome.jp/kegg-bin/get_htext?br08003.keg
- KNApSAcK Species-Metabolite Database
- Natural Product Activity and Species Source (NPASS)
- LIPID MAPSAgathisflavonehttps://lipidmaps.org/databases/lmsd/LMPK12040008Lipid Classificationhttps://www.lipidmaps.org/
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/licenseNCGC00384551-01!8-[5,7-dihydroxy-2-(4-hydroxyphenyl)-4-oxochromen-6-yl]-5,7-dihydroxy-2-(4-hydroxyphenyl)chromen-4-onehttps://mona.fiehnlab.ucdavis.edu/spectra/browse?query=exists(compound.metaData.name:%27InChIKey%27%20and%20compound.metaData.value:%27BACLASYRJRZXMY-UHFFFAOYSA-N%27)
- Metabolomics Workbench
- SpectraBaseBIS-6,8-(5,7,4'-TRIHYDROXY)-FLAVONEhttps://spectrabase.com/spectrum/5H2WRu098zWAgathisflavonehttps://spectrabase.com/spectrum/KsxFmuQyO0G
- Springer Nature
- Wikidataagathisflavonehttps://www.wikidata.org/wiki/Q27105695
- PubChem
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlagathisflavonehttps://www.ncbi.nlm.nih.gov/mesh/67079168
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 389149200https://pubchem.ncbi.nlm.nih.gov/substance/389149200
CONTENTS