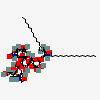
Ganglioside GM2
PubChem CID
9898635
Molecular Formula
Synonyms
- Ganglioside GM2
- GM2 lipid
- G(M2) GANGLIOSIDE
- 127663-77-8
- CHEBI:60327
Molecular Weight
1384.7 g/mol
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Dates
- Create:2006-10-25
- Modify:2025-01-11
Description
Ganglioside GM2 (18:0) is a sialotriaosylceramide that is N-acetyl-beta-D-galactosaminyl-(1->4)-alpha-N-acetylneuraminosyl-(2->3)-beta-D-galactosyl-(1->4)-beta-D-glucosyl-N-acylsphingosine in which the acyl group on the sphingosine nitrogen is octadecanoyl. A constituent of natural ganglioside GM2. It has a role as an antigen. It is a N-acetyl-beta-D-galactosaminyl-(1->4)-alpha-N-acetylneuraminosyl-(2->3)-beta-D-galactosyl-(1->4)-beta-D-glucosyl-N-acylsphingosine and a sialotriaosylceramide. It is a conjugate acid of a ganglioside GM2 (18:0) (1-).
Ganglioside GM2 is a glycosphingolipid antigen expressed by a variety of human cancer cells. GM2 containing vaccines have been shown to elicit antibodies production in melanoma patients without deleterious effects associated with an immune response to GM2. Mutations in at least 1 of 3 recessive genes: HEXA, HEXB, and GM2A cause defects in GM2 catabolism, leading to lysosomal lipid storage disorders that manifest primarily as neurodegenerative diseases, including Tay-Sachs and Sandhoff Disease.
A glycosphingolipid that accumulates due to a deficiency of hexosaminidase A or B (BETA-N-ACETYLHEXOSAMINIDASES), or GM2 activator protein, resulting in GANGLIOSIDOSES, heredity metabolic disorders that include TAY-SACHS DISEASE and SANDHOFF DISEASE.
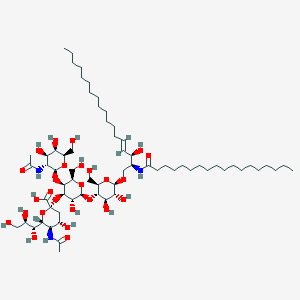
Chemical Structure Depiction
Conformer generation is disallowed since too many atoms, too flexible
(2S,4S,5R,6R)-5-acetamido-2-[(2S,3R,4R,5S,6R)-5-[(2S,3R,4R,5R,6R)-3-acetamido-4,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-2-[(2R,3S,4R,5R,6R)-4,5-dihydroxy-2-(hydroxymethyl)-6-[(E,2S,3R)-3-hydroxy-2-(octadecanoylamino)octadec-4-enoxy]oxan-3-yl]oxy-3-hydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-4-hydroxy-6-[(1R,2R)-1,2,3-trihydroxypropyl]oxane-2-carboxylic acid
Computed by LexiChem 2.6.6 (PubChem release 2019.06.18)
InChI=1S/C67H121N3O26/c1-5-7-9-11-13-15-17-19-20-22-24-26-28-30-32-34-50(80)70-43(44(77)33-31-29-27-25-23-21-18-16-14-12-10-8-6-2)40-89-64-57(85)56(84)59(48(38-73)91-64)93-65-58(86)62(60(49(39-74)92-65)94-63-52(69-42(4)76)55(83)54(82)47(37-72)90-63)96-67(66(87)88)35-45(78)51(68-41(3)75)61(95-67)53(81)46(79)36-71/h31,33,43-49,51-65,71-74,77-79,81-86H,5-30,32,34-40H2,1-4H3,(H,68,75)(H,69,76)(H,70,80)(H,87,88)/b33-31+/t43-,44+,45-,46+,47+,48+,49+,51+,52+,53+,54-,55+,56+,57+,58+,59+,60-,61+,62+,63-,64+,65-,67-/m0/s1
Computed by InChI 1.0.5 (PubChem release 2019.06.18)
GIVLTTJNORAZON-HDBOBKCLSA-N
Computed by InChI 1.0.5 (PubChem release 2019.06.18)
CCCCCCCCCCCCCCCCCC(=O)N[C@@H](CO[C@H]1[C@@H]([C@H]([C@@H]([C@H](O1)CO)O[C@H]2[C@@H]([C@H]([C@H]([C@H](O2)CO)O[C@H]3[C@@H]([C@H]([C@H]([C@H](O3)CO)O)O)NC(=O)C)O[C@@]4(C[C@@H]([C@H]([C@@H](O4)[C@@H]([C@@H](CO)O)O)NC(=O)C)O)C(=O)O)O)O)O)[C@@H](/C=C/CCCCCCCCCCCCC)O
Computed by OEChem 2.3.0 (PubChem release 2024.12.12)
C67H121N3O26
Computed by PubChem 2.1 (PubChem release 2019.06.18)
- G(M2) Ganglioside
- Ganglioside GM2
- Ganglioside, GM2
- Ganglioside, Tay-Sachs Disease
- GM2 Ganglioside
- GM2, Ganglioside
- Tay Sachs Disease Ganglioside
- Tay-Sachs Disease Ganglioside
- Ganglioside GM2
- GM2 lipid
- G(M2) GANGLIOSIDE
- 127663-77-8
- CHEBI:60327
- (2S,3R,4E)-3-hydroxy-2-(octadecanoylamino)octadec-4-en-1-yl 2-acetamido-2-deoxy-beta-D-galactopyranosyl-(1->4)-[5-acetamido-3,5-dideoxy-D-glycero-alpha-D-galacto-non-2-ulopyranonosyl-(2->3)]-beta-D-galactopyranosyl-(1->4)-beta-D-glucopyranoside
- beta-D-GalNAc-(1->4)-[alpha-Neu5Ac-(2->3)]-beta-D-Gal-(1->4)-beta-D-Glc-(1<->1)-N-octadecanoylsphingosine
- monosialoganglioside GM2
- Epitope ID:139972
- ganglioside GM2 (18:0)
- SCHEMBL21683088
- ganglioside GM2 (18:1/18:0)
- DA-53487
- PD006807
- HY-148385
- CS-0621068
- beta-D-GalNAc-(1->4)-[alpha-Neu5Ac-(2->3)]-beta-D-Gal-(1->4)-beta-D-Glc-(1<->1)-N-stearoylsphingosine
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
1384.7 g/mol
Reference
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Property Name
XLogP3-AA
Property Value
5.6
Reference
Computed by XLogP3 3.0 (PubChem release 2019.06.18)
Property Name
Hydrogen Bond Donor Count
Property Value
17
Reference
Computed by Cactvs 3.4.6.11 (PubChem release 2019.06.18)
Property Name
Hydrogen Bond Acceptor Count
Property Value
26
Reference
Computed by Cactvs 3.4.6.11 (PubChem release 2019.06.18)
Property Name
Rotatable Bond Count
Property Value
49
Reference
Computed by Cactvs 3.4.6.11 (PubChem release 2019.06.18)
Property Name
Exact Mass
Property Value
1383.82383097 Da
Reference
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Property Name
Monoisotopic Mass
Property Value
1383.82383097 Da
Reference
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Property Name
Topological Polar Surface Area
Property Value
461 Ų
Reference
Computed by Cactvs 3.4.6.11 (PubChem release 2019.06.18)
Property Name
Heavy Atom Count
Property Value
96
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
2180
Reference
Computed by Cactvs 3.4.6.11 (PubChem release 2019.06.18)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
23
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
1
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
1
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2019.01.04)
Lipids -> Sphingolipids [SP] -> Acidic glycosphingolipids [SP06] -> Gangliosides [SP0601]
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Same Connectivity Count
Same Stereo Count
Same Isotope Count
Same Parent, Connectivity Count
Same Parent, Stereo Count
Same Parent, Isotope Count
Same Parent, Exact Count
Mixtures, Components, and Neutralized Forms Count
Similar Compounds (2D)
Similar Conformers (3D)
New Zealand EPA Inventory of Chemical Status
Ganglioside GM2: Does not have an individual approval but may be used under an appropriate group standard
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/Ganglioside GM2https://commonchemistry.cas.org/detail?cas_rn=104443-57-4Ganglioside GM2https://commonchemistry.cas.org/detail?cas_rn=19600-01-2
- New Zealand Environmental Protection Authority (EPA)LICENSEThis work is licensed under the Creative Commons Attribution-ShareAlike 4.0 International licence.https://www.epa.govt.nz/about-this-site/general-copyright-statement/
- ChEBIGanglioside GM2 (18:0)https://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:60327
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuse
- Comparative Toxicogenomics Database (CTD)LICENSEIt is to be used only for research and educational purposes. Any reproduction or use for commercial purpose is prohibited without the prior express written permission of NC State University.http://ctdbase.org/about/legal.jspG(M2) Gangliosidehttps://ctdbase.org/detail.go?type=chem&acc=D005678
- LIPID MAPSHex(2)-HexNAc-NeuAc-Cer 36:1;O2https://lipidmaps.org/databases/lmsd/LMSP0601AM02Lipid Classificationhttps://www.lipidmaps.org/
- WikidataGM2 (ganglioside)https://www.wikidata.org/wiki/Q5513693
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlG(M2) Gangliosidehttps://www.ncbi.nlm.nih.gov/mesh/68005678
- PubChem
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
CONTENTS