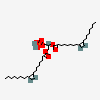
Dioleoylphosphatidic acid
PubChem CID
9547172
Molecular Formula
Synonyms
- Dioleoylphosphatidic acid
- Dioleoyl phosphatidic acid
- CHEBI:60427
- 1,2-dioleoylglycerol-3-phosphate
- 1,2-Dioleoyl-sn-glycero-3-phosphate
Molecular Weight
701.0 g/mol
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Dates
- Create:2006-08-30
- Modify:2024-12-28
Description
Dioleoyl phosphatidic acid is a phosphatidic acid in which the phosphatidyl acyl groups are both oleoyl. It is a conjugate acid of a dioleoylphosphatidate(2-).
Chemical Structure Depiction
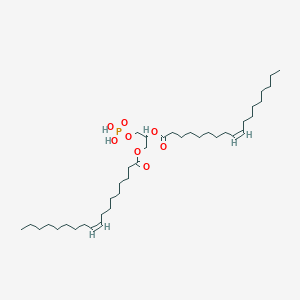
Conformer generation is disallowed since too flexible
IUPAC
O1,O2-dioleoyl-O3-phosphono-rac-glycerol
[2-[(Z)-octadec-9-enoyl]oxy-3-phosphonooxypropyl] (Z)-octadec-9-enoate
Computed by LexiChem 2.6.6 (PubChem release 2019.06.18)
InChI=1S/C39H73O8P/c1-3-5-7-9-11-13-15-17-19-21-23-25-27-29-31-33-38(40)45-35-37(36-46-48(42,43)44)47-39(41)34-32-30-28-26-24-22-20-18-16-14-12-10-8-6-4-2/h17-20,37H,3-16,21-36H2,1-2H3,(H2,42,43,44)/b19-17-,20-18-
Computed by InChI 1.0.5 (PubChem release 2019.06.18)
MHUWZNTUIIFHAS-CLFAGFIQSA-N
Computed by InChI 1.0.5 (PubChem release 2019.06.18)
CCCCCCCC/C=C\CCCCCCCC(=O)OCC(COP(=O)(O)O)OC(=O)CCCCCCC/C=C\CCCCCCCC
Computed by OEChem 2.1.5 (PubChem release 2019.06.18)
C39H73O8P
Computed by PubChem 2.1 (PubChem release 2019.06.18)
- 1,2-dioleoyl-sn-glycero-3-phosphate
- dioleoylphosphatidic acid
- Dioleoylphosphatidic acid
- Dioleoyl phosphatidic acid
- CHEBI:60427
- 1,2-dioleoylglycerol-3-phosphate
- 1,2-Dioleoyl-sn-glycero-3-phosphate
- PA(18:1/18:1)[U]
- PA(18:1(9Z)/18:1(9Z))[U]
- [2-[(Z)-octadec-9-enoyl]oxy-3-phosphonooxypropyl] (Z)-octadec-9-enoate
- Epitope ID:136905
- GTPL5512
- SCHEMBL3817083
- EX-A8496
- PA 18:1_18:1
- Q27077072
- 3-(phosphonooxy)propane-1,2-diyl (9Z,9'Z)bis-octadec-9-enoate
- [1-[(E)-octadec-9-enoyl]oxy-3-phosphonooxypropan-2-yl] (E)-octadec-9-enoate
- [1-[(Z)-octadec-9-enoyl]oxy-3-phosphonooxypropan-2-yl] (Z)-octadec-9-enoate
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
701.0 g/mol
Reference
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Property Name
XLogP3-AA
Property Value
13.2
Reference
Computed by XLogP3 3.0 (PubChem release 2019.06.18)
Property Name
Hydrogen Bond Donor Count
Property Value
2
Reference
Computed by Cactvs 3.4.6.11 (PubChem release 2019.06.18)
Property Name
Hydrogen Bond Acceptor Count
Property Value
8
Reference
Computed by Cactvs 3.4.6.11 (PubChem release 2019.06.18)
Property Name
Rotatable Bond Count
Property Value
38
Reference
Computed by Cactvs 3.4.6.11 (PubChem release 2019.06.18)
Property Name
Exact Mass
Property Value
700.50430628 Da
Reference
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Property Name
Monoisotopic Mass
Property Value
700.50430628 Da
Reference
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Property Name
Topological Polar Surface Area
Property Value
119Ų
Reference
Computed by Cactvs 3.4.6.11 (PubChem release 2019.06.18)
Property Name
Heavy Atom Count
Property Value
48
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
838
Reference
Computed by Cactvs 3.4.6.11 (PubChem release 2019.06.18)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
1
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
2
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
1
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2019.01.04)
259.86 Ų [M-H]- [CCS Type: TW; Method: calibrated with phosphatidylcholines (ESI+) and phosphatidylethanolamines (ESI-) doubly charged cardiolipins calibrated with poly-DL-alanine]
MoNA ID
MS Category
In-Silico
MS Type
Other
MS Level
MS2
Precursor Type
[M-H]-
Precursor m/z
699.49649
Instrument
SCIEX 5600
Instrument Type
in-silico QTOF
Ionization Mode
negative
Collision Energy
45 V
Retention Time
6.09
Top 5 Peaks
281.2479 100
699.4965 40.04
152.9958 30.03
417.2408 10.01
435.2513 10.01
MoNA ID
MS Category
In-Silico
MS Type
Other
Precursor Type
[M-H]-
Precursor m/z
699.49703
Ionization Mode
negative
Retention Time
8.76
Top 5 Peaks
281.2479 100
699.497 40.04
152.9958 30.03
435.2513 20.02
417.2408 20.02
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Same Connectivity Count
Same Parent, Connectivity Count
Same Parent, Exact Count
Mixtures, Components, and Neutralized Forms Count
Similar Compounds (2D)
Similar Conformers (3D)
PubMed Count
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=MHUWZNTUIIFHAS-CLFAGFIQSA-N
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/Dioleoyl phosphatidic acidhttps://commonchemistry.cas.org/detail?cas_rn=14268-17-8
- CCSbaseCCSbase Classificationhttps://ccsbase.net/
- ChEBIDioleoyl phosphatidic acidhttps://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:60427
- IUPHAR/BPS Guide to PHARMACOLOGYLICENSEThe Guide to PHARMACOLOGY database is licensed under the Open Data Commons Open Database License (ODbL) https://opendatacommons.org/licenses/odbl/. Its contents are licensed under a Creative Commons Attribution-ShareAlike 4.0 International License (http://creativecommons.org/licenses/by-sa/4.0/)https://www.guidetopharmacology.org/about.jsp#licensedioleoylphosphatidic acidhttps://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5512Guide to Pharmacology Target Classificationhttps://www.guidetopharmacology.org/targets.jsp
- Therapeutic Target Database (TTD)dioleoylphosphatidic acidhttps://idrblab.net/ttd/data/drug/details/D07KBZ
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- PharosLICENSEData accessed from Pharos and TCRD is publicly available from the primary sources listed above. Please respect their individual licenses regarding proper use and redistribution.https://pharos.nih.gov/aboutdioleoylphosphatidic acidhttps://pharos.nih.gov/ligands/C164U96MRVSB
- WikidataDioleoylphosphatidic acidhttps://www.wikidata.org/wiki/Q27077072
- PubChem
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmldioleoylphosphatidic acidhttps://www.ncbi.nlm.nih.gov/mesh/67037657
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 402787570https://pubchem.ncbi.nlm.nih.gov/substance/402787570
CONTENTS