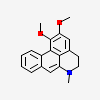
Dehydronuciferine
PubChem CID
821347
Molecular Formula
Synonyms
- Dehydronuciferine
- 7630-74-2
- Dehydronuciferin
- 5,6-Dihydro-1,2-dimethoxy-6-methyl-4H-dibenzo[de,g]quinoline
- 1,2-Dimethoxy-6-methyl-5,6-dihydro-4H-dibenzo[de,g]quinoline
Molecular Weight
293.4 g/mol
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Dates
- Create:2005-07-09
- Modify:2025-01-04
Description
Dehydronuciferine is an isoquinoline alkaloid.
Dehydronuciferine has been reported in Nelumbo nucifera with data available.
Chemical Structure Depiction
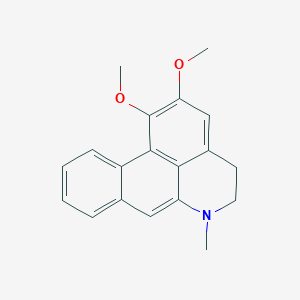
15,16-dimethoxy-10-methyl-10-azatetracyclo[7.7.1.02,7.013,17]heptadeca-1(17),2,4,6,8,13,15-heptaene
Computed by LexiChem 2.6.6 (PubChem release 2019.06.18)
InChI=1S/C19H19NO2/c1-20-9-8-13-11-16(21-2)19(22-3)18-14-7-5-4-6-12(14)10-15(20)17(13)18/h4-7,10-11H,8-9H2,1-3H3
Computed by InChI 1.0.5 (PubChem release 2019.06.18)
JBGSWIBJAGBGOP-UHFFFAOYSA-N
Computed by InChI 1.0.5 (PubChem release 2019.06.18)
CN1CCC2=CC(=C(C3=C2C1=CC4=CC=CC=C43)OC)OC
Computed by OEChem 2.3.0 (PubChem release 2024.12.12)
C19H19NO2
Computed by PubChem 2.1 (PubChem release 2019.06.18)
- Dehydronuciferine
- 7630-74-2
- Dehydronuciferin
- 5,6-Dihydro-1,2-dimethoxy-6-methyl-4H-dibenzo[de,g]quinoline
- 1,2-Dimethoxy-6-methyl-5,6-dihydro-4H-dibenzo[de,g]quinoline
- 15,16-dimethoxy-10-methyl-10-azatetracyclo[7.7.1.02,7.013,17]heptadeca-1(17),2,4,6,8,13,15-heptaene
- 15,16-dimethoxy-10-methyl-10-azatetracyclo[7.7.1.0(2),?.0(1)(3),(1)?]heptadeca-1(17),2(7),3,5,8,13,15-heptaene
- Nuciferine, 10a,11-dehydro-
- Y87HRZ3K29
- CHEMBL2316501
- CHEBI:174097
- DTXSID801183819
- HY-N4261
- NSC785154
- ZB1875
- AKOS037515219
- NSC-785154
- AC-34648
- DA-52413
- MS-24198
- Aporphine, 6a,7-didehydro-1,2-dimethoxy-
- CS-0032561
- F82186
- AE-508/21135042
- 4H-Dibenzo[de,g]quinoline, 5,6-dihydro-1,2-dimethoxy-6-methyl-
- 15,16-dimethoxy-10-methyl-10-azatetracyclo[7.7.1.0^{2,7}.0^{13,17}]heptadeca-1(16),2,4,6,8,13(17),14-heptaene
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
293.4 g/mol
Reference
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Property Name
XLogP3-AA
Property Value
4.5
Reference
Computed by XLogP3 3.0 (PubChem release 2019.06.18)
Property Name
Hydrogen Bond Donor Count
Property Value
0
Reference
Computed by Cactvs 3.4.6.11 (PubChem release 2019.06.18)
Property Name
Hydrogen Bond Acceptor Count
Property Value
3
Reference
Computed by Cactvs 3.4.6.11 (PubChem release 2019.06.18)
Property Name
Rotatable Bond Count
Property Value
2
Reference
Computed by Cactvs 3.4.6.11 (PubChem release 2019.06.18)
Property Name
Exact Mass
Property Value
293.141578849 Da
Reference
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Property Name
Monoisotopic Mass
Property Value
293.141578849 Da
Reference
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Property Name
Topological Polar Surface Area
Property Value
21.7Ų
Reference
Computed by Cactvs 3.4.6.11 (PubChem release 2019.06.18)
Property Name
Heavy Atom Count
Property Value
22
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
401
Reference
Computed by Cactvs 3.4.6.11 (PubChem release 2019.06.18)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
1
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2011.04.04)
Solid
130 - 131 °C
MoNA ID
MS Category
Experimental
MS Type
Other
MS Level
MS2
Precursor Type
[M+H]+
Precursor m/z
294.14913615903106
Ionization Mode
positive
Retention Time
5.48
Top 5 Peaks
236.10785014366442 100
264.1029542134861 71.85
235.09975249557792 65.93
279.1302964781133 65.19
218.1013584784344 45.56
MoNA ID
MS Category
Experimental
MS Type
Other
MS Level
MS2
Precursor Type
[M+H]+
Precursor m/z
294.1486705558649
Ionization Mode
positive
Retention Time
5.54
Top 5 Peaks
279.1255798339844 100
294.1488342285156 98.03
264.1023254394531 44.41
263.1305236816406 41.14
236.1071014404297 34.26
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Similar Compounds (2D)
Similar Conformers (3D)
Same Count
PubMed Count
Membrane
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=JBGSWIBJAGBGOP-UHFFFAOYSA-N
The LOTUS Initiative for Open Natural Products Research: frozen dataset union wikidata (with metadata) | DOI:10.5281/zenodo.5794106
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/5,6-Dihydro-1,2-dimethoxy-6-methyl-4H-dibenzo[de,g]quinolinehttps://commonchemistry.cas.org/detail?cas_rn=7630-74-2
- EPA DSSTox5,6-Dihydro-1,2-dimethoxy-6-methyl-4H-dibenzo[de,g]quinolinehttps://comptox.epa.gov/dashboard/DTXSID801183819CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingDehydronuciferinehttps://gsrs.ncats.nih.gov/ginas/app/beta/substances/Y87HRZ3K29
- Human Metabolome Database (HMDB)LICENSEHMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications.http://www.hmdb.ca/citingDehydronuciferinehttp://www.hmdb.ca/metabolites/HMDB0033737
- ChEBIDehydronuciferinehttps://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:174097
- LOTUS - the natural products occurrence databaseLICENSEThe code for LOTUS is released under the GNU General Public License v3.0.https://lotus.nprod.net/Dehydronuciferinehttps://www.wikidata.org/wiki/Q105124323LOTUS Treehttps://lotus.naturalproducts.net/
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.html
- FooDBLICENSEFooDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (FooDB) and the original publication.https://foodb.ca/aboutDehydronuciferinehttps://foodb.ca/compounds/FDB011863
- Japan Chemical Substance Dictionary (Nikkaji)
- KNApSAcK Species-Metabolite Database
- Natural Product Activity and Species Source (NPASS)Dehydronuciferinehttps://bidd.group/NPASS/compound.php?compoundID=NPC285941
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- Metabolomics Workbench
- Thieme ChemistryLICENSEThe Thieme Chemistry contribution within PubChem is provided under a CC-BY-NC-ND 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc-nd/4.0/
- WikidataDehydronuciferinehttps://www.wikidata.org/wiki/Q105124323
- PubChem
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 389241179https://pubchem.ncbi.nlm.nih.gov/substance/389241179
CONTENTS