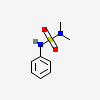Sulfamide, N,N-dimethyl-N'-phenyl-
PubChem CID
78441
Molecular Formula
Synonyms
- N,N-Dimethyl-N'-phenylsulfamide
- 4710-17-2
- (dimethylsulfamoylamino)benzene
- Sulfamide, N,N-dimethyl-N'-phenyl-
- N-(Dimethylsulfamoyl)aniline
Molecular Weight
200.26 g/mol
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Dates
- Create:2005-03-27
- Modify:2025-01-11
Description
N,N-dimethyl-N'-phenylsulfamide is a member of the class of sulfamides that is N-phenylsulfuric diamide substituted by two methyl groups at the amino nitrogen atom. It is a metabolite of the agrochemical dichlofluanid. It has a role as a marine xenobiotic metabolite.
Chemical Structure Depiction
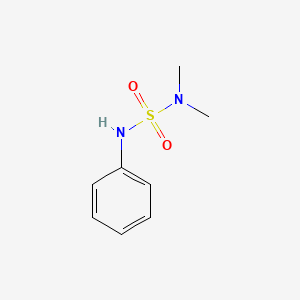
(dimethylsulfamoylamino)benzene
Computed by Lexichem TK 2.7.0 (PubChem release 2021.10.14)
InChI=1S/C8H12N2O2S/c1-10(2)13(11,12)9-8-6-4-3-5-7-8/h3-7,9H,1-2H3
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
QCDQDISRALTLNQ-UHFFFAOYSA-N
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
CN(C)S(=O)(=O)NC1=CC=CC=C1
Computed by OEChem 2.3.0 (PubChem release 2024.12.12)
C8H12N2O2S
Computed by PubChem 2.2 (PubChem release 2021.10.14)
- N,N-Dimethyl-N'-phenylsulfamide
- 4710-17-2
- (dimethylsulfamoylamino)benzene
- Sulfamide, N,N-dimethyl-N'-phenyl-
- N-(Dimethylsulfamoyl)aniline
- BRN 2805076
- N,N-Dimethyl-N'-phenylsulphamide
- EINECS 225-198-8
- N,N-dimethyl-N'-phenylsulfuric diamide
- dimethyl(phenylsulfamoyl)amine
- CHEBI:83467
- DTXSID60197041
- N-(Dimethylsulfamoyl)aniline-d5
- 3-12-00-01118 (Beilstein Handbook Reference)
- 1246819-81-7
- Dimethylaminosulfanilid
- dimethylaminosulfanilide
- Dichlofluanid metabolite
- Maybridge1_005058
- N,N-Dimethylaminosulfanilide
- SCHEMBL460093
- HMS555N20
- DTXCID70119532
- 9F78F3X5G3
- N,N-Dimethyl-N'-phenylsulfamide #
- STK068645
- AKOS000646572
- CCG-247591
- CS-0327223
- NS00000267
- W-109993
- Q27156850
- N,N-Dimethyl-N inverted exclamation marka-phenylsulfamide
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
200.26 g/mol
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
XLogP3
Property Value
0.9
Reference
Computed by XLogP3 3.0 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Donor Count
Property Value
1
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Acceptor Count
Property Value
4
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Rotatable Bond Count
Property Value
3
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Exact Mass
Property Value
200.06194880 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Monoisotopic Mass
Property Value
200.06194880 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Topological Polar Surface Area
Property Value
57.8 Ų
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Heavy Atom Count
Property Value
13
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
237
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
1
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2021.10.14)
Semi-standard non-polar
1626.1
Environmental transformation -> Pesticide transformation products (metabolite, successor)
S60 | SWISSPEST19 | Swiss Pesticides and Metabolites from Kiefer et al 2019 | DOI:10.5281/zenodo.3544759
Pesticides -> Fungicides -> Amide fungicides -> Phenylsulfamide fungicides -> Transformation products
S66 | EAWAGTPS | Parent-Transformation Product Pairs from Eawag | DOI:10.5281/zenodo.3754448
Pesticide -> transformation product
S120 | DUSTCT2024 | Substances from Second NORMAN Collaborative Dust Trial | DOI:10.5281/zenodo.13835254
NIST Number
240227
Library
Main library
Total Peaks
70
m/z Top Peak
200
m/z 2nd Highest
45
m/z 3rd Highest
92
Thumbnail
NIST Number
403305
Library
Replicate library
Total Peaks
42
m/z Top Peak
200
m/z 2nd Highest
45
m/z 3rd Highest
92
Thumbnail
Accession ID
Authors
Nikiforos Alygizakis, Nikolaos Thomaidis, University of Athens
Instrument
Bruker maXis Impact
Instrument Type
LC-ESI-QTOF
MS Level
MS2
Ionization Mode
POSITIVE
Ionization
ESI
Collision Energy
10 eV
Fragmentation Mode
CID
Column Name
Acclaim RSLC C18 2.2um, 2.1x100mm, Thermo
Retention Time
6.1 min
Precursor m/z
201.0692
Precursor Adduct
[M+H]+
Top 5 Peaks
201.0685 999
137.1063 827
202.071 87
138.1097 64
203.0659 39
License
CC BY-SA
Accession ID
Authors
Nikiforos Alygizakis, Nikolaos Thomaidis, University of Athens
Instrument
Bruker maXis Impact
Instrument Type
LC-ESI-QTOF
MS Level
MS2
Ionization Mode
POSITIVE
Ionization
ESI
Collision Energy
20 eV
Fragmentation Mode
CID
Column Name
Acclaim RSLC C18 2.2um, 2.1x100mm, Thermo
Retention Time
6.1 min
Precursor m/z
201.0692
Precursor Adduct
[M+H]+
Top 5 Peaks
137.1066 999
122.0827 474
121.088 151
136.0998 125
201.0684 119
License
CC BY-SA
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Same Connectivity Count
Same Parent, Connectivity Count
Similar Compounds (2D)
Similar Conformers (3D)
Same Count
Pesticides -> Fungicides -> Amide fungicides -> Phenylsulfamide fungicides -> Transformation products
S66 | EAWAGTPS | Parent-Transformation Product Pairs from Eawag | DOI:10.5281/zenodo.3754448
Dimethylaminosulfanilide is a known environmental transformation product of Dichlofluanid.
S60 | SWISSPEST19 | Swiss Pesticides and Metabolites from Kiefer et al 2019 | DOI:10.5281/zenodo.3544759
N,N-Dimethylaminosulfanilide is a known environmental transformation product of Dichlofluanid.
S66 | EAWAGTPS | Parent-Transformation Product Pairs from Eawag | DOI:10.5281/zenodo.3754448
Pesticides -> Fungicides -> Amide fungicides -> Phenylsulfamide fungicides -> Transformation products
S66 | EAWAGTPS | Parent-Transformation Product Pairs from Eawag | DOI:10.5281/zenodo.3754448
Environmental transformation -> Pesticide transformation products (metabolite, successor)
S60 | SWISSPEST19 | Swiss Pesticides and Metabolites from Kiefer et al 2019 | DOI:10.5281/zenodo.3544759
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=QCDQDISRALTLNQ-UHFFFAOYSA-N
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/N,N-Dimethyl-N′-phenylsulfamidehttps://commonchemistry.cas.org/detail?cas_rn=4710-17-2
- ChemIDplusSulfamide, N,N-dimethyl-N'-phenyl-https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0004710172ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA DSSToxSulfamide, N,N-dimethyl-N'-phenyl-https://comptox.epa.gov/dashboard/DTXSID60197041CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-noticeN,N-dimethyl-N'-phenylsulphamidehttps://echa.europa.eu/substance-information/-/substanceinfo/100.022.907
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking(Dimethylsulfamoylamino)benzenehttps://gsrs.ncats.nih.gov/ginas/app/beta/substances/9F78F3X5G3
- ChEBIN,N-dimethyl-N'-phenylsulfamidehttps://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:83467
- Japan Chemical Substance Dictionary (Nikkaji)
- MassBank EuropeN,N-Dimethyl-N'-phenylsulfamidehttps://massbank.eu/MassBank/Result.jsp?inchikey=QCDQDISRALTLNQ-UHFFFAOYSA-N
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- NIST Mass Spectrometry Data CenterLICENSEData covered by the Standard Reference Data Act of 1968 as amended.https://www.nist.gov/srd/public-lawSulfamide, N,N-dimethyl-N'-phenyl-http://www.nist.gov/srd/nist1a.cfm
- SpectraBase
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/N,N-DimethylaminosulfanilideNORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- Springer Nature
- WikidataN,N-dimethyl-N'-phenylsulfamidehttps://www.wikidata.org/wiki/Q27156850
- PubChem
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 403633922https://pubchem.ncbi.nlm.nih.gov/substance/403633922
CONTENTS