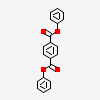
Diphenyl terephthalate
PubChem CID
73757
Molecular Formula
Synonyms
- Diphenyl terephthalate
- 1539-04-4
- Terephthalic acid, diphenyl ester
- 1,4-diphenyl benzene-1,4-dicarboxylate
- DIPHENYLTEREPHTHALATE
Molecular Weight
318.3 g/mol
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Dates
- Create:2005-03-26
- Modify:2024-12-28
Chemical Structure Depiction

diphenyl benzene-1,4-dicarboxylate
Computed by Lexichem TK 2.7.0 (PubChem release 2021.10.14)
InChI=1S/C20H14O4/c21-19(23-17-7-3-1-4-8-17)15-11-13-16(14-12-15)20(22)24-18-9-5-2-6-10-18/h1-14H
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
HPGJOUYGWKFYQW-UHFFFAOYSA-N
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
C1=CC=C(C=C1)OC(=O)C2=CC=C(C=C2)C(=O)OC3=CC=CC=C3
Computed by OEChem 2.3.0 (PubChem release 2021.10.14)
C20H14O4
Computed by PubChem 2.2 (PubChem release 2021.10.14)
- Diphenyl terephthalate
- 1539-04-4
- Terephthalic acid, diphenyl ester
- 1,4-diphenyl benzene-1,4-dicarboxylate
- DIPHENYLTEREPHTHALATE
- diphenyl benzene-1,4-dicarboxylate
- 1,4-BENZENEDICARBOXYLIC ACID, DIPHENYL ESTER
- MFCD00016574
- S0KW8M264E
- 1,4-Benzenedicarboxylic acid, 1,4-diphenyl ester
- NSC-402474
- UNII-S0KW8M264E
- EINECS 216-264-7
- NSC 402474
- SCHEMBL70034
- terephthalic acid diphenyl ester
- DTXSID8061765
- AC9154
- NSC402474
- AKOS030573904
- DIPHENYL 1,4-BENZENEDICARBOXYLATE
- AS-57824
- SY237720
- 1ST163329
- 1ST163329-400T
- DB-254535
- CS-0156093
- NS00025000
- Diphenyl terephthalate Solution in Toluene, 400mug/mL
- Q27288399
- F1916-0148
- Terephthalic acid, bis-phenyl ester 100 microg/mL in Hexane
- 1,4-Benzenedicarboxylic acid, 1,4-diphenyl ester; 1,4-Benzenedicarboxylic acid, diphenyl ester (9CI); Terephthalic acid, diphenyl ester (6CI,8CI); Diphenyl 1,4-benzenedicarboxylate; Diphenyl terephthalate; NSC 402474
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
318.3 g/mol
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
XLogP3
Property Value
5
Reference
Computed by XLogP3 3.0 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Donor Count
Property Value
0
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Acceptor Count
Property Value
4
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Rotatable Bond Count
Property Value
6
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Exact Mass
Property Value
318.08920892 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Monoisotopic Mass
Property Value
318.08920892 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Topological Polar Surface Area
Property Value
52.6Ų
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Heavy Atom Count
Property Value
24
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
373
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
1
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2021.10.14)
White odorless powder; [Alfa Aesar MSDS]
Plastics & Rubber -> Other Plasticizers
NIST Number
133725
Library
Main library
Total Peaks
48
m/z Top Peak
225
m/z 2nd Highest
104
m/z 3rd Highest
226
Thumbnail
NIST Number
324070
Library
Replicate library
Total Peaks
25
m/z Top Peak
225
m/z 2nd Highest
104
m/z 3rd Highest
226
Thumbnail
Instrument Name
Bruker MultiRAM Stand Alone FT-Raman Spectrometer
Technique
FT-Raman
Source of Spectrum
Bio-Rad Laboratories, Inc.
Source of Sample
Alfa Aesar, Thermo Fisher Scientific
Catalog Number
L02877
Lot Number
10116536
Copyright
Copyright © 2017-2024 John Wiley & Sons, Inc. All Rights Reserved.
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Same Parent, Exact Count
Mixtures, Components, and Neutralized Forms Count
Similar Compounds (2D)
Similar Conformers (3D)
EPA TSCA Commercial Activity Status
1,4-Benzenedicarboxylic acid, 1,4-diphenyl ester: INACTIVE
An oxidizer that may cause fire on contact with combustible materials; An irritant; Targets the nerves and lungs; [Sigma-Aldrich MSDS] See Bis(2-ethylhexyl) terephthalate.
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=HPGJOUYGWKFYQW-UHFFFAOYSA-N
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/1,4-Diphenyl 1,4-benzenedicarboxylatehttps://commonchemistry.cas.org/detail?cas_rn=1539-04-4
- ChemIDplusDiphenyl terephthalatehttps://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0001539044ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- DTP/NCILICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuse
- EPA Chemicals under the TSCA1,4-Benzenedicarboxylic acid, 1,4-diphenyl esterhttps://www.epa.gov/chemicals-under-tscaEPA TSCA Classificationhttps://www.epa.gov/tsca-inventory
- EPA DSSToxDiphenyl terephthalatehttps://comptox.epa.gov/dashboard/DTXSID8061765CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-noticeDiphenyl terephthalatehttps://echa.europa.eu/substance-information/-/substanceinfo/100.014.786
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingDIPHENYL TEREPHTHALATEhttps://gsrs.ncats.nih.gov/ginas/app/beta/substances/S0KW8M264E
- Haz-Map, Information on Hazardous Chemicals and Occupational DiseasesLICENSECopyright (c) 2022 Haz-Map(R). All rights reserved. Unless otherwise indicated, all materials from Haz-Map are copyrighted by Haz-Map(R). No part of these materials, either text or image may be used for any purpose other than for personal use. Therefore, reproduction, modification, storage in a retrieval system or retransmission, in any form or by any means, electronic, mechanical or otherwise, for reasons other than personal use, is strictly prohibited without prior written permission.https://haz-map.com/AboutDiphenyl terephthalatehttps://haz-map.com/Agents/20638
- Japan Chemical Substance Dictionary (Nikkaji)
- NIST Mass Spectrometry Data CenterLICENSEData covered by the Standard Reference Data Act of 1968 as amended.https://www.nist.gov/srd/public-lawDiphenyl terephthalatehttp://www.nist.gov/srd/nist1a.cfm
- SpectraBaseDIPHENYL TEREPHTHALATEhttps://spectrabase.com/spectrum/C8PZFcFXXjf1,4-Benzenedicarboxylic acid, diphenyl ester Benzene-1,4-dioic acid, diphenyl esterhttps://spectrabase.com/spectrum/2vnkdv6KmlQTEREPHTHALIC ACID, DIPHENYL ESTERhttps://spectrabase.com/spectrum/Ev6rh4l8mRuDIPHENYL TEREPHTHALATEhttps://spectrabase.com/spectrum/La4ISZgL2r4Diphenyl terephthalatehttps://spectrabase.com/spectrum/JNjMXAeGXQoBenzene-1,4-dioic acid, diphenyl esterhttps://spectrabase.com/spectrum/rKWeiE0wWGDiphenyl terephthalatehttps://spectrabase.com/spectrum/226Fkr0HMig
- Springer Nature
- SpringerMaterialsTerephthalic acid diphenyl esterhttps://materials.springer.com/substanceprofile/docs/smsid_arovscpavtszjnxu
- Wikidatadiphenyl terephthalatehttps://www.wikidata.org/wiki/Q27288399
- PubChem
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/NORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- EPA Substance Registry ServicesEPA SRS List Classificationhttps://sor.epa.gov/sor_internet/registry/substreg/LandingPage.do
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 403386400https://pubchem.ncbi.nlm.nih.gov/substance/403386400
CONTENTS