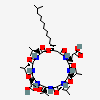
Surfactin D
PubChem CID
70789015
Molecular Formula
Synonyms
- surfactin D
- CHEBI:71979
- 3-[(3S,6R,9S,12S,15R,18S,21S,25R)-9-(carboxymethyl)-3,6,15,18-tetraisobutyl-12-isopropyl-25-(11-methyldodecyl)-2,5,8,11,14,17,20,23-octaoxo-1-oxa-4,7,10,13,16,19,22-heptaazacyclopentacosan-21-yl]propanoic acid
- 3-((3S,6R,9S,12S,15R,18S,21S,25R)-9-(carboxymethyl)-25-(11-methyldodecyl)-3,6,15,18-tetrakis(2-methylpropyl)-2,5,8,11,14,17,20,23-octaoxo-12-propan-2-yl-1-oxa-4,7,10,13,16,19,22-heptazacyclopentacos-21-yl)propanoic acid
- 3-((3S,6R,9S,12S,15R,18S,21S,25R)-9-(carboxymethyl)-3,6,15,18-tetraisobutyl-12-isopropyl-25-(11-methyldodecyl)-2,5,8,11,14,17,20,23-octaoxo-1-oxa-4,7,10,13,16,19,22-heptaazacyclopentacosan-21-yl)propanoic acid
Molecular Weight
1050.4 g/mol
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Dates
- Create:2013-03-05
- Modify:2024-12-07
Description
Surfactin D is a cyclodepsipeptide that is N-[(3R)-3-hydroxy-14-methylpentadecanoyl]-L-alpha-glutamyl-L-leucyl-D-leucyl-L-valyl-L-alpha-aspartyl-D-leucyl-L-leucine in which the C-terminal carboxy group has been lactonised by condensation with the alcoholic hydroxy group. It has a role as an antibacterial agent, an antifungal agent, an antiviral agent, a surfactant, a metabolite and an antineoplastic agent. It is a cyclodepsipeptide, a lipopeptide antibiotic and a macrocyclic lactone.
surfactin D has been reported in Bacillus subtilis with data available.
Chemical Structure Depiction
Conformer generation is disallowed since too many atoms, too flexible
SVG Image

IUPAC Condensed
cyclo[Asp-D-Leu-Leu-ObAla(3R-isotridecyl)-Glu-Leu-D-Leu-Val]
HELM
PEPTIDE1{D.[dL].L.[*C(=O)C[C@@H](CCCCCCCCCCC(C)C)O* |$_R2;;;;;;;;;;;;;;;;;;;_R1$|].E.L.[dL].V}$PEPTIDE1,PEPTIDE1,8:R2-1:R1$$$
IUPAC
cyclo[L-alpha-aspartyl-D-leucyl-L-leucyl-N-oxa-(3R)-3-isotridecyl-beta-alanyl-L-alpha-glutamyl-L-leucyl-D-leucyl-L-valyl]
3-[(3S,6R,9S,12S,15R,18S,21S,25R)-9-(carboxymethyl)-25-(11-methyldodecyl)-3,6,15,18-tetrakis(2-methylpropyl)-2,5,8,11,14,17,20,23-octaoxo-12-propan-2-yl-1-oxa-4,7,10,13,16,19,22-heptazacyclopentacos-21-yl]propanoic acid
Computed by LexiChem 2.6.6 (PubChem release 2019.06.18)
InChI=1S/C54H95N7O13/c1-31(2)21-19-17-15-13-14-16-18-20-22-37-29-44(62)55-38(23-24-45(63)64)48(67)56-39(25-32(3)4)49(68)58-41(27-34(7)8)52(71)61-47(36(11)12)53(72)59-42(30-46(65)66)51(70)57-40(26-33(5)6)50(69)60-43(28-35(9)10)54(73)74-37/h31-43,47H,13-30H2,1-12H3,(H,55,62)(H,56,67)(H,57,70)(H,58,68)(H,59,72)(H,60,69)(H,61,71)(H,63,64)(H,65,66)/t37-,38+,39+,40-,41-,42+,43+,47+/m1/s1
Computed by InChI 1.0.5 (PubChem release 2019.06.18)
HPBKRTWLUQRMNA-PTYDTDROSA-N
Computed by InChI 1.0.5 (PubChem release 2019.06.18)
CC(C)CCCCCCCCCC[C@@H]1CC(=O)N[C@H](C(=O)N[C@H](C(=O)N[C@@H](C(=O)N[C@H](C(=O)N[C@H](C(=O)N[C@@H](C(=O)N[C@H](C(=O)O1)CC(C)C)CC(C)C)CC(=O)O)C(C)C)CC(C)C)CC(C)C)CCC(=O)O
Computed by OEChem 2.1.5 (PubChem release 2019.06.18)
C54H95N7O13
Computed by PubChem 2.1 (PubChem release 2019.06.18)
- surfactin D
- CHEBI:71979
- 3-[(3S,6R,9S,12S,15R,18S,21S,25R)-9-(carboxymethyl)-3,6,15,18-tetraisobutyl-12-isopropyl-25-(11-methyldodecyl)-2,5,8,11,14,17,20,23-octaoxo-1-oxa-4,7,10,13,16,19,22-heptaazacyclopentacosan-21-yl]propanoic acid
- 3-((3S,6R,9S,12S,15R,18S,21S,25R)-9-(carboxymethyl)-25-(11-methyldodecyl)-3,6,15,18-tetrakis(2-methylpropyl)-2,5,8,11,14,17,20,23-octaoxo-12-propan-2-yl-1-oxa-4,7,10,13,16,19,22-heptazacyclopentacos-21-yl)propanoic acid
- 3-((3S,6R,9S,12S,15R,18S,21S,25R)-9-(carboxymethyl)-3,6,15,18-tetraisobutyl-12-isopropyl-25-(11-methyldodecyl)-2,5,8,11,14,17,20,23-octaoxo-1-oxa-4,7,10,13,16,19,22-heptaazacyclopentacosan-21-yl)propanoic acid
- 3-[(3S,6R,9S,12S,15R,18S,21S,25R)-9-(carboxymethyl)-25-(11-methyldodecyl)-3,6,15,18-tetrakis(2-methylpropyl)-2,5,8,11,14,17,20,23-octaoxo-12-propan-2-yl-1-oxa-4,7,10,13,16,19,22-heptazacyclopentacos-21-yl]propanoic acid
- Q27139840
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
1050.4 g/mol
Reference
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Property Name
XLogP3-AA
Property Value
9.4
Reference
Computed by XLogP3 3.0 (PubChem release 2019.06.18)
Property Name
Hydrogen Bond Donor Count
Property Value
9
Reference
Computed by Cactvs 3.4.6.11 (PubChem release 2019.06.18)
Property Name
Hydrogen Bond Acceptor Count
Property Value
13
Reference
Computed by Cactvs 3.4.6.11 (PubChem release 2019.06.18)
Property Name
Rotatable Bond Count
Property Value
25
Reference
Computed by Cactvs 3.4.6.11 (PubChem release 2019.06.18)
Property Name
Exact Mass
Property Value
1049.69878611 Da
Reference
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Property Name
Monoisotopic Mass
Property Value
1049.69878611 Da
Reference
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Property Name
Topological Polar Surface Area
Property Value
305Ų
Reference
Computed by Cactvs 3.4.6.11 (PubChem release 2019.06.18)
Property Name
Heavy Atom Count
Property Value
74
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
1820
Reference
Computed by Cactvs 3.4.6.11 (PubChem release 2019.06.18)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
8
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
1
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2019.01.04)
MoNA ID
MS Category
Experimental
MS Type
LC-MS
MS Level
MS2
Precursor Type
[M+H]+
Precursor m/z
1050.71
Instrument
Orbitrap
Ionization Mode
positive
Top 5 Peaks
86.096596 100
199.180603 30.18
201.123871 19.90
227.175964 19.58
84.044640 18.80
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Same Connectivity Count
Same Parent, Connectivity Count
Similar Compounds (2D)
Similar Conformers (3D)
Same Count
The LOTUS Initiative for Open Natural Products Research: frozen dataset union wikidata (with metadata) | DOI:10.5281/zenodo.5794106
- ChEBI
- LOTUS - the natural products occurrence databaseLICENSEThe code for LOTUS is released under the GNU General Public License v3.0.https://lotus.nprod.net/surfactin Dhttps://www.wikidata.org/wiki/Q27139840LOTUS Treehttps://lotus.naturalproducts.net/
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- Metabolomics Workbench
- Wikidatasurfactin Dhttps://www.wikidata.org/wiki/Q27139840
- PubChem
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
CONTENTS