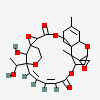
Satratoxin G
PubChem CID
6437354
Molecular Formula
Synonyms
- Satratoxin G
- isosatratoxin G
- BRN 4623147
- 2',3'-Dihydro-2',3'-epoxysatratoxin H
- Satratoxin H, 2',3'-dihydro-2',3'-epoxy-
Molecular Weight
544.6 g/mol
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Dates
- Create:2006-04-28
- Modify:2025-01-04
Chemical Structure Depiction
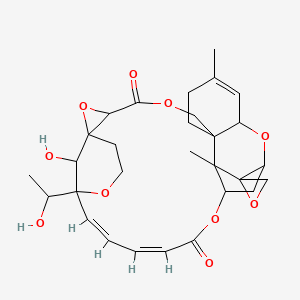
Conformer generation is disallowed since too many undefined stereo centers
(20Z,22E)-28-hydroxy-24-(1-hydroxyethyl)-10,16-dimethylspiro[2,5,13,18,25-pentaoxahexacyclo[22.3.1.114,17.01,3.07,12.07,16]nonacosa-10,20,22-triene-15,2'-oxirane]-4,19-dione
Computed by Lexichem TK 2.7.0 (PubChem release 2021.05.07)
InChI=1S/C29H36O10/c1-16-7-9-26-14-34-23(32)22-28(39-22)10-11-35-27(17(2)30,24(28)33)8-5-4-6-21(31)38-18-13-20(37-19(26)12-16)29(15-36-29)25(18,26)3/h4-6,8,12,17-20,22,24,30,33H,7,9-11,13-15H2,1-3H3/b6-4-,8-5+
Computed by InChI 1.0.6 (PubChem release 2021.05.07)
GTONGKBINDTWOM-QXMOYCCXSA-N
Computed by InChI 1.0.6 (PubChem release 2021.05.07)
CC1=CC2C3(CC1)COC(=O)C4C5(O4)CCOC(C5O)(/C=C/C=C\C(=O)OC6C3(C7(CO7)C(C6)O2)C)C(C)O
Computed by OEChem 2.3.0 (PubChem release 2024.12.12)
C29H36O10
Computed by PubChem 2.1 (PubChem release 2021.05.07)
54385-60-3, 73513-00-5
- isosatratoxin G
- satratoxin G
- Satratoxin G
- isosatratoxin G
- BRN 4623147
- 2',3'-Dihydro-2',3'-epoxysatratoxin H
- Satratoxin H, 2',3'-dihydro-2',3'-epoxy-
- 53126-63-9
- Spiro(10,12:19,22a-dimethano-4H,5H,22aH-oxireno(8,9)(1,6,12)trioxacyclooctadecino(3,4-d)(1)benzopyran-11(10H),2'-oxirane)-2,14(1aH,19H)-dione, 6,8a,11a,12,21,22-hexahydro-23-hydroxy-19-(1-hydroxyethyl)-7,11a-dimethyl-
- DTXSID50891844
- DTXCID501031265
- AKOS040753941
- NS00094916
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
544.6 g/mol
Reference
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Property Name
XLogP3-AA
Property Value
1.2
Reference
Computed by XLogP3 3.0 (PubChem release 2021.05.07)
Property Name
Hydrogen Bond Donor Count
Property Value
2
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.05.07)
Property Name
Hydrogen Bond Acceptor Count
Property Value
10
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.05.07)
Property Name
Rotatable Bond Count
Property Value
1
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.05.07)
Property Name
Exact Mass
Property Value
544.23084734 Da
Reference
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Property Name
Monoisotopic Mass
Property Value
544.23084734 Da
Reference
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Property Name
Topological Polar Surface Area
Property Value
137 Ų
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.05.07)
Property Name
Heavy Atom Count
Property Value
39
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
1190
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.05.07)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
11
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
2
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
1
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2019.01.04)
Accession ID
Authors
Justin B. Renaud, Mark W. Sumarah, Agriculture and Agri-Food Canada
Instrument
Q-Exactive Orbitrap Thermo Scientific
Instrument Type
LC-ESI-ITFT
MS Level
MS2
Ionization Mode
POSITIVE
Ionization
ESI
Collision Energy
10(NCE)
Fragmentation Mode
HCD
Column Name
Agilent RRHD Eclipse 50 x 2 mm, 1.8 uM
Retention Time
3.18
Precursor m/z
545.2376
Precursor Adduct
[M+H]+
Top 5 Peaks
545.2381 999
231.138 59
249.1485 37
License
CC BY-SA
Reference
Renaud, J. B.; Sumarah, M. W. Data Independent Acquisition-Digital Archiving Mass Spectrometry: Application to Single Kernel Mycotoxin Analysis of Fusarium Graminearum Infected Maize. Analytical and Bioanalytical Chemistry 2016, 408 (12), 3083-91. DOI:10.1007/s00216-016-9391-5
Accession ID
Authors
Justin B. Renaud, Mark W. Sumarah, Agriculture and Agri-Food Canada
Instrument
Q-Exactive Orbitrap Thermo Scientific
Instrument Type
LC-ESI-ITFT
MS Level
MS2
Ionization Mode
POSITIVE
Ionization
ESI
Collision Energy
20(NCE)
Fragmentation Mode
HCD
Column Name
Agilent RRHD Eclipse 50 x 2 mm, 1.8 uM
Retention Time
3.18
Precursor m/z
545.2376
Precursor Adduct
[M+H]+
Top 5 Peaks
545.2381 999
231.138 684
249.1485 383
213.1274 172
105.0699 164
License
CC BY-SA
Reference
Renaud, J. B.; Sumarah, M. W. Data Independent Acquisition-Digital Archiving Mass Spectrometry: Application to Single Kernel Mycotoxin Analysis of Fusarium Graminearum Infected Maize. Analytical and Bioanalytical Chemistry 2016, 408 (12), 3083-91. DOI:10.1007/s00216-016-9391-5
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Same Connectivity Count
Same Parent, Connectivity Count
Similar Compounds (2D)
Similar Conformers (3D)
Same Count
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/
- ChemIDplusChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- MassBank Europe
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- WikidataSatratoxin Ghttps://www.wikidata.org/wiki/Q81987557
- PubChem
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlsatratoxin Ghttps://www.ncbi.nlm.nih.gov/mesh/67028268
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/NORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- NCBI
CONTENTS