Piperyline
- Piperyline
- Trichostachine
- Trichostachin
- Piperiline
- Piperylin
- Create:2005-03-27
- Modify:2025-01-18
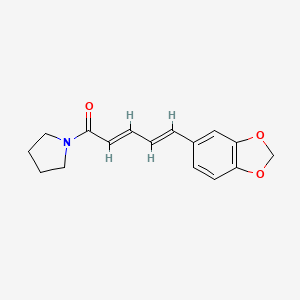
- (2E,4E)-5-(1,3-Benzodioxol-5-yl)-1-(1-pyrrolidinyl)-2,4-pentadien-1-one
- 1-piperoylpyrrolidine
- piperyline
- Piperyline
- Trichostachine
- Trichostachin
- Piperiline
- Piperylin
- Pyrroperine
- 25924-78-1
- Pyrrolidine, 1-piperoyl-, (E,E)-
- UNII-GV0493SM38
- (E,E)-Trichostachine
- GV0493SM38
- CHEBI:9691
- DTXSID20180595
- (2E,4E)-5-(2H-1,3-benzodioxol-5-yl)-1-(pyrrolidin-1-yl)penta-2,4-dien-1-one
- 2E,4E)-5-(2H-1,3-benzodioxol-5-yl)-1-(pyrrolidin-1-yl)penta-2,4-dien-1-one
- PYRROLIDINE, 1-((2E,4E)-5-(1,3-BENZODIOXOL-5-YL)-1-OXO-2,4-PENTADIENYL)-
- 1-(5-(1,3-Benzodioxol-5-yl)-1-oxo-2,4-pentadienyl)pyrrolidine
- Pyrrolidine, 1-(5-(1,3-benzodioxol-5-yl)-1-oxo-2,4-pentadienyl)-
- Piperyline, analytical standard
- MEGxp0_000203
- 1-Piperoyl-(E,E)-Pyrrolidine
- CHEMBL1087296
- N-[10-(13,14-Methylenedioxyphenyl)-7(E),9(Z)-pentadienoyl]-pyrrolidine
- SCHEMBL16274586
- ACon1_000155
- DTXCID60103086
- (2E,4E)-5-(1,3-benzodioxol-5-yl)-1-pyrrolidin-1-ylpenta-2,4-dien-1-one
- Pyrrolidine, 1-[5-(1,3-benzodioxol-5-yl)-1-oxo-2,4-pentadienyl]-, (E,E)-
- NCGC00180828-01
- 54976-54-4
- PIPERYLINE (CONSTITUENT OF BLACK PEPPER)
- BRD-K64601708-001-01-6
- PIPERYLINE (CONSTITUENT OF BLACK PEPPER) [DSC]
- Q27108470
- 1-[(2E,4E)-5-(1,3-benzodioxol-5-yl)-1-oxo-2,4-pentadienyl]pyrrolidine
- 1-[(2E,4E)-5-(1,3-Benzodioxol-5-yl)-2,4-pentadienoyl]pyrrolidine #
- 5-(2H-1,3-Benzodioxol-5-yl)-1-(pyrrolidin-1-yl)penta-2,4-dien-1-one
- (2E,4E)-5-(1,3-benzodioxol-5-yl)-1-(1-pyrrolidinyl)-2,4-pentadien-1-one
- (2E,4E)-5-(1,3-benzodioxol-5-yl)-1-(pyrrolidin-1-yl)penta-2,4-dien-1-one
- (2E,4E)-5-(Benzo[d][1,3]dioxol-5-yl)-1-(pyrrolidin-1-yl)penta-2,4-dien-1-one
- (2E,4E)-5-(benzo[d][1,3]dioxol-5-yl)-1-(pyrrolidin-1-yl)penta-2,4-diene-1-one
- Chemical shift
- Chemical structure
- Clearing parameter
- Crystal structure
- Crystal-to-crystal transition
- Density
- Formula unit
- Formula weight
- Gross formula
- Liquid crystalline phase
- Melting temperature
- Melting transition
- Molecular structure
- Nuclear magnetic resonance
- Solid-to-solid transition
- Space group
- Spin-spin coupling constant
- Transition pressure
- Unit cell
- Unit cell parameter
201.0 99.99
271.0 79.70
172.0 19.40
272.0 19
200.0 17.50
201 99.99
271 79.70
172 19.40
272 19
200 17.50
201.055099 35664
135.044678 18408
115.05439 14016
143.049255 11536
171.044235 9536
201.055099 100
135.044678 51.62
115.05439 39.30
143.049255 32.35
171.044235 26.74
201.055099 100
135.044678 51.62
115.054390 39.30
143.049255 32.35
171.044235 26.74
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=GQIJYUMTOUBHSH-IJIVKGSJSA-N
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/
- ChemIDplusPyrrolidine, 1-(5-(1,3-benzodioxol-5-yl)-1-oxo-2,4-pentadienyl)-https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0054976544ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA DSSToxCompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Human Metabolome Database (HMDB)LICENSEHMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications.http://www.hmdb.ca/citing(E,E)-Trichostachinehttp://www.hmdb.ca/metabolites/HMDB0029374HMDB0029374_cms_27004https://hmdb.ca/metabolites/HMDB0029374#spectra
- ChEBI
- LOTUS - the natural products occurrence databaseLICENSEThe code for LOTUS is released under the GNU General Public License v3.0.https://lotus.nprod.net/Trichostachinehttps://www.wikidata.org/wiki/Q27108470LOTUS Treehttps://lotus.naturalproducts.net/
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- Crystallography Open Database (COD)LICENSEAll data in the COD and the database itself are dedicated to the public domain and licensed under the CC0 License. Users of the data should acknowledge the original authors of the structural data.https://creativecommons.org/publicdomain/zero/1.0/
- FooDBLICENSEFooDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (FooDB) and the original publication.https://foodb.ca/about(E,E)-Trichostachinehttps://foodb.ca/compounds/FDB000444
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- NIST Mass Spectrometry Data CenterLICENSEData covered by the Standard Reference Data Act of 1968 as amended.https://www.nist.gov/srd/public-lawPyrrolidine, 1-[5-(1,3-benzodioxol-5-yl)-1-oxo-2,4-pentadienyl]-, (E,E)-http://www.nist.gov/srd/nist1a.cfm
- SpectraBase(2E,4E)-5-(1,3-benzodioxol-5-yl)-1-(1-pyrrolidinyl)-1-penta-2,4-dienonehttps://spectrabase.com/spectrum/B0rdI4LMuU8PIPERILINE;(2E,4E)-N-[5-(3',4'-METHYLENEDIOXY-PHENYL)-PENTA-2,4-DIENOYL]-PYRROLIDINEhttps://spectrabase.com/spectrum/DenMr4Uo98f
- Japan Chemical Substance Dictionary (Nikkaji)
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.html
- KNApSAcK Species-Metabolite Database
- Natural Product Activity and Species Source (NPASS)
- Metabolomics Workbench(E,E)-Trichostachinehttps://www.metabolomicsworkbench.org/data/StructureData.php?RegNo=43798
- Rhea - Annotated Reactions DatabaseLICENSERhea has chosen to apply the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0/). This means that you are free to copy, distribute, display and make commercial use of the database in all legislations, provided you credit (cite) Rhea.https://www.rhea-db.org/help/license-disclaimer
- SpringerMaterials
- Thieme ChemistryLICENSEThe Thieme Chemistry contribution within PubChem is provided under a CC-BY-NC-ND 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc-nd/4.0/
- Wikidatapiperylinehttps://www.wikidata.org/wiki/Q27108470
- PubChem
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.html
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 388343099https://pubchem.ncbi.nlm.nih.gov/substance/388343099