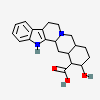
17-Hydroxyyohimban-16-carboxylic acid
PubChem CID
630921
Molecular Formula
Synonyms
- 17-Hydroxyyohimban-16-carboxylic acid
- Oprea1_780636
- SCHEMBL1691213
- Yohimban-16-carboxylic acid, 17-hydroxy-, (16.alpha.,17.alpha.)-
- DTXSID00859454
Molecular Weight
340.4 g/mol
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Dates
- Create:2005-03-28
- Modify:2025-01-18
See also:  Yohimbic Acid (annotation moved to).
Yohimbic Acid (annotation moved to).
 Yohimbic Acid (annotation moved to).
Yohimbic Acid (annotation moved to).Chemical Structure Depiction
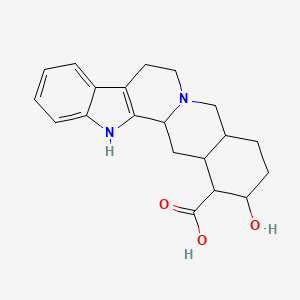
18-hydroxy-1,3,11,12,14,15,16,17,18,19,20,21-dodecahydroyohimban-19-carboxylic acid
Computed by Lexichem TK 2.7.0 (PubChem release 2021.05.07)
InChI=1S/C20H24N2O3/c23-17-6-5-11-10-22-8-7-13-12-3-1-2-4-15(12)21-19(13)16(22)9-14(11)18(17)20(24)25/h1-4,11,14,16-18,21,23H,5-10H2,(H,24,25)
Computed by InChI 1.0.6 (PubChem release 2021.05.07)
AADVZSXPNRLYLV-UHFFFAOYSA-N
Computed by InChI 1.0.6 (PubChem release 2021.05.07)
C1CC(C(C2C1CN3CCC4=C(C3C2)NC5=CC=CC=C45)C(=O)O)O
Computed by OEChem 2.3.0 (PubChem release 2024.12.12)
C20H24N2O3
Computed by PubChem 2.1 (PubChem release 2021.05.07)
- 17-Hydroxyyohimban-16-carboxylic acid
- Oprea1_780636
- SCHEMBL1691213
- Yohimban-16-carboxylic acid, 17-hydroxy-, (16.alpha.,17.alpha.)-
- DTXSID00859454
- STL372879
- AKOS025247987
- 17-Hydroxyyohimban-16-carboxylic acid #
- EN300-717855
- 18-hydroxy-3,13-diazapentacyclo[11.8.0.0^{2,10}.0^{4,9}.0^{15,20}]henicosa-2(10),4(9),5,7-tetraene-19-carboxylic acid
- 875845-00-4
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
340.4 g/mol
Reference
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Property Name
XLogP3
Property Value
0.3
Reference
Computed by XLogP3 3.0 (PubChem release 2021.05.07)
Property Name
Hydrogen Bond Donor Count
Property Value
3
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.05.07)
Property Name
Hydrogen Bond Acceptor Count
Property Value
4
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.05.07)
Property Name
Rotatable Bond Count
Property Value
1
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.05.07)
Property Name
Exact Mass
Property Value
340.17869263 Da
Reference
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Property Name
Monoisotopic Mass
Property Value
340.17869263 Da
Reference
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Property Name
Topological Polar Surface Area
Property Value
76.6 Ų
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.05.07)
Property Name
Heavy Atom Count
Property Value
25
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
541
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.05.07)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
5
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
1
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2019.01.04)
NIST Number
238573
Library
Main library
Total Peaks
307
m/z Top Peak
339
m/z 2nd Highest
340
m/z 3rd Highest
169
Thumbnail
Accession ID
Authors
Tetsuya Mori, Center for Sustainable Resource Science, RIKEN
Instrument
LC, Waters Acquity UPLC System; MS, Waters Xevo G2 Q-Tof
Instrument Type
LC-ESI-QTOF
MS Level
MS2
Ionization Mode
POSITIVE
Ionization
ESI
Collision Energy
6V
Column Name
Acquity bridged ethyl hybrid C18 (1.7 um, 2.1 mm * 100 mm, Waters)
Retention Time
3.8201
Precursor m/z
341.1859691
Precursor Adduct
[M+H]+
Top 5 Peaks
341.1857 999
144.08035 534
198.11302 151
145.08478 60
210.11324 32
License
CC BY-NC-SA
Reference
Tsugawa H., Nakabayashi R., Mori T., Yamada Y., Takahashi M., Rai A., Sugiyama R., Yamamoto H., Nakaya T., Yamazaki M., Kooke R., Bac-Molenaar JA., Oztolan-Erol N., Keurentjes JJB., Arita M., Saito K. (2019) "A cheminformatics approach to characterize metabolomes in stable-isotope-labeled organisms" Nature Methods 16(4):295-298. [doi:10.1038/s41592-019-0358-2]
Accession ID
Authors
Tetsuya Mori, Center for Sustainable Resource Science, RIKEN
Instrument
LC, Waters Acquity UPLC System; MS, Waters Xevo G2 Q-Tof
Instrument Type
LC-ESI-QTOF
MS Level
MS2
Ionization Mode
POSITIVE
Ionization
ESI
Collision Energy
6V
Column Name
Acquity bridged ethyl hybrid C18 (1.7 um, 2.1 mm * 100 mm, Waters)
Retention Time
3.8201
Precursor m/z
341.1859691
Precursor Adduct
[M+H]+
Top 5 Peaks
341.18689 999
341.23959 32
198.11275 11
144.08057 8
License
CC BY-NC-SA
Reference
Tsugawa H., Nakabayashi R., Mori T., Yamada Y., Takahashi M., Rai A., Sugiyama R., Yamamoto H., Nakaya T., Yamazaki M., Kooke R., Bac-Molenaar JA., Oztolan-Erol N., Keurentjes JJB., Arita M., Saito K. (2019) "A cheminformatics approach to characterize metabolomes in stable-isotope-labeled organisms" Nature Methods 16(4):295-298. [doi:10.1038/s41592-019-0358-2]
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Same Connectivity Count
Same Parent, Connectivity Count
Same Parent, Exact Count
Mixtures, Components, and Neutralized Forms Count
Similar Compounds (2D)
Similar Conformers (3D)
 Yohimbic Acid (annotation moved to)
Yohimbic Acid (annotation moved to)- EPA DSSTox17-Hydroxyyohimban-16-carboxylic acidhttps://comptox.epa.gov/dashboard/DTXSID00859454CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- MassBank Europe
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- NIST Mass Spectrometry Data CenterLICENSEData covered by the Standard Reference Data Act of 1968 as amended.https://www.nist.gov/srd/public-lawYohimbic Acidhttp://www.nist.gov/srd/nist1a.cfm
- PubChem
CONTENTS