Isoreserpine
PubChem CID
5701996
Molecular Formula
Synonyms
- Isoreserpine
- (-)-Isoreserpine
- 482-85-9
- 3-Epireserpine
- 3-Isoreserpine
Molecular Weight
608.7 g/mol
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Dates
- Create:2006-03-07
- Modify:2025-01-18
Description
(1S,15S,17R,18R,19S,20S)-6,18-dimethoxy-17-[oxo-(3,4,5-trimethoxyphenyl)methoxy]-1,3,11,12,14,15,16,17,18,19,20,21-dodecahydroyohimban-19-carboxylic acid methyl ester is a yohimban alkaloid.
Isoreserpine has been reported in Rauvolfia grandiflora and Rauvolfia tetraphylla with data available.
Chemical Structure Depiction
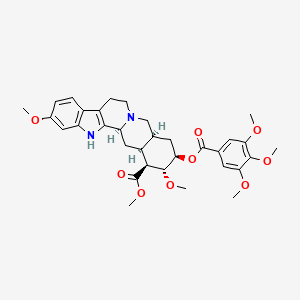
methyl (1S,15S,17R,18R,19S,20S)-6,18-dimethoxy-17-(3,4,5-trimethoxybenzoyl)oxy-1,3,11,12,14,15,16,17,18,19,20,21-dodecahydroyohimban-19-carboxylate
Computed by Lexichem TK 2.7.0 (PubChem release 2021.10.14)
InChI=1S/C33H40N2O9/c1-38-19-7-8-20-21-9-10-35-16-18-13-27(44-32(36)17-11-25(39-2)30(41-4)26(12-17)40-3)31(42-5)28(33(37)43-6)22(18)15-24(35)29(21)34-23(20)14-19/h7-8,11-12,14,18,22,24,27-28,31,34H,9-10,13,15-16H2,1-6H3/t18-,22+,24+,27-,28+,31+/m1/s1
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
QEVHRUUCFGRFIF-VPHNHGCZSA-N
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
CO[C@H]1[C@@H](C[C@@H]2CN3CCC4=C([C@@H]3C[C@@H]2[C@@H]1C(=O)OC)NC5=C4C=CC(=C5)OC)OC(=O)C6=CC(=C(C(=C6)OC)OC)OC
Computed by OEChem 2.3.0 (PubChem release 2024.12.12)
C33H40N2O9
Computed by PubChem 2.2 (PubChem release 2021.10.14)
482-85-9
- Isoreserpine
- (-)-Isoreserpine
- 482-85-9
- 3-Epireserpine
- 3-Isoreserpine
- NSC 80138
- DTXSID50858952
- methyl (1S,15S,17R,18R,19S,20S)-6,18-dimethoxy-17-(3,4,5-trimethoxybenzoyl)oxy-1,3,11,12,14,15,16,17,18,19,20,21-dodecahydroyohimban-19-carboxylate
- 20alpha-Yohimban-16beta-carboxylic acid, 18beta-hydroxy-11,17alpha-dimethoxy-, methyl ester, 3,4,5-trimethoxybenzoate (ester) (8CI)
- Yohimban-16-carboxylic acid, 11,17-dimethoxy-18-((3,4,5-trimethoxybenzoyl)oxy)-, methyl ester, (16beta,17alpha,18beta,20alpha)-
- 20alpha-Yohimban-16beta-carboxylic acid, 18beta-hydroxy-11,17alpha-dimethoxy-, methyl ester, 3,4,5-trimethoxybenzoate (ester)
- NSC80138
- Spectrum_000004
- Spectrum2_001770
- Spectrum3_001236
- Spectrum5_000924
- BSPBio_002712
- KBioSS_000344
- SPECTRUM300534
- methyl dimethoxy-(3,4,5-trimethoxybenzoyl)oxy-[?]carboxylate
- DivK1c_000200
- SCHEMBL731599
- SPBio_001639
- CHEMBL388848
- BCBcMAP01_000242
- CHEBI:92652
- HMS500J22
- KBio1_000200
- KBio2_000344
- KBio2_002912
- KBio2_005480
- KBio3_002212
- NINDS_000200
- DTXCID201324366
- CCG-39604
- AKOS040752126
- IDI1_000200
- SMP1_000292
- R0007
- EN300-19736137
- BRD-K37080523-001-01-0
- Q27164362
- (1S,15S,17R,18R,19S,20S)-6,18-dimethoxy-17-[oxo-(3,4,5-trimethoxyphenyl)methoxy]-1,3,11,12,14,15,16,17,18,19,20,21-dodecahydroyohimban-19-carboxylic acid methyl ester
- methyl (1S,15S,17R,18R,19S,20S)-6,18-dimethoxy-17-(3,4,5-trimethoxybenzoyloxy)-3,13-diazapentacyclo[11.8.0.0^{2,10}.0^{4,9}.0^{15,20}]henicosa-2(10),4(9),5,7-tetraene-19-carboxylate
- Yohimban-16-carboxylic acid, 11,17-dimethoxy-18-((3,4,5-trimethoxybenzoyl)oxy)-, methyl ester, (16beta,17alpha,18beta,20alpha)-(9CI)
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
608.7 g/mol
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
XLogP3-AA
Property Value
4
Reference
Computed by XLogP3 3.0 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Donor Count
Property Value
1
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Acceptor Count
Property Value
10
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Rotatable Bond Count
Property Value
10
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Exact Mass
Property Value
608.27338086 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Monoisotopic Mass
Property Value
608.27338086 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Topological Polar Surface Area
Property Value
118 Ų
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Heavy Atom Count
Property Value
44
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
1000
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
6
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
1
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2021.10.14)
Accession ID
Authors
Tetsuya Mori, Center for Sustainable Resource Science, RIKEN
Instrument
LC, Waters Acquity UPLC System; MS, Waters Xevo G2 Q-Tof
Instrument Type
LC-ESI-QTOF
MS Level
MS2
Ionization Mode
POSITIVE
Ionization
ESI
Collision Energy
6V
Column Name
Acquity bridged ethyl hybrid C18 (1.7 um, 2.1 mm * 100 mm, Waters)
Retention Time
6.11695
Precursor m/z
609.2806573
Precursor Adduct
[M+H]+
Top 5 Peaks
609.28082 999
195.06598 172
174.09169 95
397.21182 92
448.19479 85
License
CC BY-NC-SA
Reference
Tsugawa H., Nakabayashi R., Mori T., Yamada Y., Takahashi M., Rai A., Sugiyama R., Yamamoto H., Nakaya T., Yamazaki M., Kooke R., Bac-Molenaar JA., Oztolan-Erol N., Keurentjes JJB., Arita M., Saito K. (2019) "A cheminformatics approach to characterize metabolomes in stable-isotope-labeled organisms" Nature Methods 16(4):295-298. [doi:10.1038/s41592-019-0358-2]
Accession ID
Authors
Tetsuya Mori, Center for Sustainable Resource Science, RIKEN
Instrument
LC, Waters Acquity UPLC System; MS, Waters Xevo G2 Q-Tof
Instrument Type
LC-ESI-QTOF
MS Level
MS2
Ionization Mode
POSITIVE
Ionization
ESI
Collision Energy
6V
Column Name
Acquity bridged ethyl hybrid C18 (1.7 um, 2.1 mm * 100 mm, Waters)
Retention Time
6.11695
Precursor m/z
609.2806573
Precursor Adduct
[M+H]+
Top 5 Peaks
609.28229 999
609.35541 19
License
CC BY-NC-SA
Reference
Tsugawa H., Nakabayashi R., Mori T., Yamada Y., Takahashi M., Rai A., Sugiyama R., Yamamoto H., Nakaya T., Yamazaki M., Kooke R., Bac-Molenaar JA., Oztolan-Erol N., Keurentjes JJB., Arita M., Saito K. (2019) "A cheminformatics approach to characterize metabolomes in stable-isotope-labeled organisms" Nature Methods 16(4):295-298. [doi:10.1038/s41592-019-0358-2]
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Same Connectivity Count
Same Stereo Count
Same Isotope Count
Same Parent, Connectivity Count
Same Parent, Stereo Count
Same Parent, Isotope Count
Same Parent, Exact Count
Mixtures, Components, and Neutralized Forms Count
Similar Compounds (2D)
Similar Conformers (3D)
PubMed Count
The LOTUS Initiative for Open Natural Products Research: frozen dataset union wikidata (with metadata) | DOI:10.5281/zenodo.5794106
- ChEBI(1S,15S,17R,18R,19S,20S)-6,18-dimethoxy-17-[oxo-(3,4,5-trimethoxyphenyl)methoxy]-1,3,11,12,14,15,16,17,18,19,20,21-dodecahydroyohimban-19-carboxylic acid methyl esterhttps://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:92652
- LOTUS - the natural products occurrence databaseLICENSEThe code for LOTUS is released under the GNU General Public License v3.0.https://lotus.nprod.net/Isoreserpinehttps://www.wikidata.org/wiki/Q27164362LOTUS Treehttps://lotus.naturalproducts.net/
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- ChemIDplusChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA DSSToxIsoreserpinehttps://comptox.epa.gov/dashboard/DTXSID50858952CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- Japan Chemical Substance Dictionary (Nikkaji)
- MassBank Europe
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- SpectraBase(-)-Isoreserpinehttps://spectrabase.com/spectrum/LEAPJubhR5q(-)-Isoreserpinehttps://spectrabase.com/spectrum/5HFgxIEHU4j(-)-Isoreserpinehttps://spectrabase.com/spectrum/9psAsUEpx4L
- Springer Nature
- WikidataIsoreserpinehttps://www.wikidata.org/wiki/Q27164362
- PubChem
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- NCBI
CONTENTS