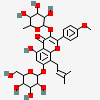
Icarin
- Icarin
- Icariine
- 5-hydroxy-2-(4-methoxyphenyl)-8-(3-methylbut-2-enyl)-7-[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-3-(3,4,5-trihydroxy-6-methyloxan-2-yl)oxychromen-4-one
- Icarrin
- SCHEMBL7738609
- Create:2005-03-26
- Modify:2025-01-11
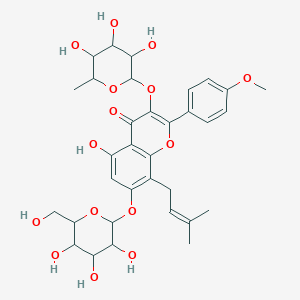
- 2-(4'-methoxyphenyl)-3-rhamnosido-5-hydroxyl-7-glucosido-8-(3'-methyl-2-butylenyl)-4-chromanone
- icarrin
- Icarin
- Icariine
- 5-hydroxy-2-(4-methoxyphenyl)-8-(3-methylbut-2-enyl)-7-[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-3-(3,4,5-trihydroxy-6-methyloxan-2-yl)oxychromen-4-one
- Icarrin
- SCHEMBL7738609
- CHEBI:183923
- HMS3369E05
- HMS3393J13
- HMS3656K03
- NSC708926
- NSC-708926
- I0862
- 3,5-dihydroxy-4\'-methoxy-6\'\',6\'\'-dimethyl-4\'\',5\'\'-dihydropyrano[2\'\',3\'\':7,8]-flavone
- 5-hydroxy-2-(4-methoxyphenyl)-8-(3-methylbut-2-enyl)-7-[3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydropyran-2-yl]oxy-3-(3,4,5-trihydroxy-6-methyl-tetrahydropyran-2-yl)oxy-chromen-4-one
531.1825 100
369.1307 32.85
677.2399 28.10
313.0667 3.17
129.0534 1.97
369.1321 100
531.1856 58.81
313.0695 4.94
129.0535 2.97
301.0704 1.01
369.138400 100
313.066800 79.23
370.136100 20.70
531.174400 16.40
532.193700 5.37
735.260200 100
513.181000 64.32
789.216100 49.88
736.250700 41.96
514.182800 20.62
369.133897 0.83
71.048383 0.04
85.027735 0.04
313.073757 0.03
536.962096 0.02
313.09886387783956 0.79
243.08189344416456 0.07
353.1360814104495 0.02
215.0836176415512 0.02
312.0867698778396 0.02
 Icariin (annotation moved to)
Icariin (annotation moved to)Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=TZJALUIVHRYQQB-UHFFFAOYSA-N
- ChEBI
- LOTUS - the natural products occurrence databaseLICENSEThe code for LOTUS is released under the GNU General Public License v3.0.https://lotus.nprod.net/5-Hydroxy-2-(4-methoxyphenyl)-8-(3-methylbut-2-enyl)-7-[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-3-(3,4,5-trihydroxy-6-methyloxan-2-yl)oxychromen-4-onehttps://www.wikidata.org/wiki/Q105268203LOTUS Treehttps://lotus.naturalproducts.net/
- DTP/NCILICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuse
- Human Metabolome Database (HMDB)LICENSEHMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications.http://www.hmdb.ca/citing5-Hydroxy-2-(4-methoxyphenyl)-8-(3-methylbut-2-enyl)-7-[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-3-(3,4,5-trihydroxy-6-methyloxan-2-yl)oxychromen-4-onehttp://www.hmdb.ca/metabolites/HMDB0246694HMDB0246694_msms_2236013https://hmdb.ca/metabolites/HMDB0246694#spectra
- Natural Product Activity and Species Source (NPASS)
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- Wikidata5-Hydroxy-2-(4-methoxyphenyl)-8-(3-methylbut-2-enyl)-7-[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-3-(3,4,5-trihydroxy-6-methyloxan-2-yl)oxychromen-4-onehttps://www.wikidata.org/wiki/Q105268203
- PubChem
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.html
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 389836462https://pubchem.ncbi.nlm.nih.gov/substance/389836462