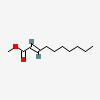
Methyl 2-decenoate
PubChem CID
5368064
Molecular Formula
Synonyms
- Methyl 2-decenoate
- methyl (E)-dec-2-enoate
- 2482-39-5
- 2-Decenoic acid, methyl ester
- Methyl (E)-2-decenoate
Molecular Weight
184.27 g/mol
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Dates
- Create:2005-03-27
- Modify:2024-12-28
Description
Methyl 2-decenoate is a fatty acid ester.
Chemical Structure Depiction
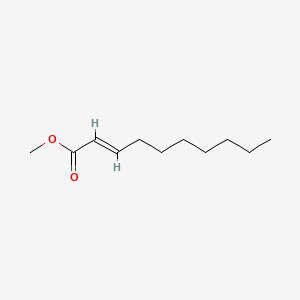
methyl (E)-dec-2-enoate
Computed by Lexichem TK 2.7.0 (PubChem release 2021.10.14)
InChI=1S/C11H20O2/c1-3-4-5-6-7-8-9-10-11(12)13-2/h9-10H,3-8H2,1-2H3/b10-9+
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
VVBWOSGRZNCEBX-MDZDMXLPSA-N
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
CCCCCCC/C=C/C(=O)OC
Computed by OEChem 2.3.0 (PubChem release 2021.10.14)
C11H20O2
Computed by PubChem 2.2 (PubChem release 2021.10.14)
2482-39-5
- Methyl 2-decenoate
- methyl (E)-dec-2-enoate
- 2482-39-5
- 2-Decenoic acid, methyl ester
- Methyl (E)-2-decenoate
- methyl dec-2-enoate
- 7367-85-3
- Methyl decenoate
- Methyl 2-decenoate, (E)-
- METHYL TRANS-2-DECENOATE
- EINECS 219-618-9
- 2-Decenoic acid, methyl ester, (E)-
- 7YEW204WFW
- AI3-36549
- Methyl T2 Decenoate
- UNII-7YEW204WFW
- Methyl (2E)-2-decenoate
- (E)-2-Decenoic acid methyl ester
- EINECS 230-916-8
- (E)-methyl 2-decenoate
- (E)-methyl dec-2-enoate
- SCHEMBL728273
- Methyl (2E)-2-decenoate #
- Methyl ester of 2-Decenoic acid
- DTXSID10179519
- CHEBI:172078
- METHYL (2E)-DEC-2-ENOATE
- LMFA07010939
- 2-Decenoic acid, methyl ester, (2E)-
- J126.505I
- NS00021930
- Q27269022
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
184.27 g/mol
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
XLogP3-AA
Property Value
4.1
Reference
Computed by XLogP3 3.0 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Donor Count
Property Value
0
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Acceptor Count
Property Value
2
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Rotatable Bond Count
Property Value
8
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Exact Mass
Property Value
184.146329876 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Monoisotopic Mass
Property Value
184.146329876 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Topological Polar Surface Area
Property Value
26.3Ų
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Heavy Atom Count
Property Value
13
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
150
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
1
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
1
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2021.10.14)
Standard non-polar
1411, 1361.5, 1299
Standard polar
1694, 1619
FLAVORING AGENT OR ADJUVANT -> FDA Substance added to food
NIST Number
53551
Library
Main library
Total Peaks
67
m/z Top Peak
87
m/z 2nd Highest
41
m/z 3rd Highest
43
Thumbnail
NIST Number
36105
Library
Main library
Total Peaks
135
m/z Top Peak
87
m/z 2nd Highest
43
m/z 3rd Highest
41
Thumbnail
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Same Connectivity Count
Same Parent, Connectivity Count
Mixtures, Components, and Neutralized Forms Count
Similar Compounds (2D)
Similar Conformers (3D)
Flavoring Agents
Substance
Used for (Technical Effect)
FLAVORING AGENT OR ADJUVANT
- Extracellular
- Membrane
Food additives -> Flavoring Agents
The Australian Inventory of Industrial Chemicals
Chemical: 2-Decenoic acid, methyl ester
New Zealand EPA Inventory of Chemical Status
2-Decenoic acid, methyl ester: Does not have an individual approval but may be used under an appropriate group standard
Chemical Assessment
Evaluation - Chemicals that are unlikely to require further regulation to manage risks to environment
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=VVBWOSGRZNCEBX-MDZDMXLPSA-N
- Australian Industrial Chemicals Introduction Scheme (AICIS)2-Decenoic acid, methyl esterhttps://services.industrialchemicals.gov.au/search-assessments/2-Decenoic acid, methyl esterhttps://services.industrialchemicals.gov.au/search-inventory/
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/Methyl (E)-2-decenoatehttps://commonchemistry.cas.org/detail?cas_rn=7367-85-3
- ChemIDplusMethyl 2-decenoate, (E)-https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0007367853ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA DSSToxMethyl 2-decenoatehttps://comptox.epa.gov/dashboard/DTXSID10179519CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-noticeMethyl 2-decenoatehttps://echa.europa.eu/substance-information/-/substanceinfo/100.017.836Methyl (E)-2-decenoatehttps://echa.europa.eu/substance-information/-/substanceinfo/100.028.106
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingMETHYL 2-DECENOATE, (E)-https://gsrs.ncats.nih.gov/ginas/app/beta/substances/7YEW204WFW
- Human Metabolome Database (HMDB)LICENSEHMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications.http://www.hmdb.ca/citingMethyl 2-decenoatehttp://www.hmdb.ca/metabolites/HMDB0032396
- New Zealand Environmental Protection Authority (EPA)LICENSEThis work is licensed under the Creative Commons Attribution-ShareAlike 4.0 International licence.https://www.epa.govt.nz/about-this-site/general-copyright-statement/
- ChEBIMethyl 2-decenoatehttps://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:172078
- EU Food Improvement AgentsMethyl dec-2-enoatehttps://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32012R0872
- FDA Substances Added to FoodLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- FooDBLICENSEFooDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (FooDB) and the original publication.https://foodb.ca/aboutMethyl 2-decenoatehttps://foodb.ca/compounds/FDB016347
- Japan Chemical Substance Dictionary (Nikkaji)
- Metabolomics WorkbenchMethyl 2-decenoatehttps://www.metabolomicsworkbench.org/data/StructureData.php?RegNo=45486
- NIST Mass Spectrometry Data CenterLICENSEData covered by the Standard Reference Data Act of 1968 as amended.https://www.nist.gov/srd/public-law2-Decenoic acid, methyl ester, (E)-http://www.nist.gov/srd/nist1a.cfm
- SpectraBase2-DECENOIC ACID, METHYL ESTER, (E)-https://spectrabase.com/spectrum/GLu8Ud7ym62-DECENOIC ACID, METHYL ESTERhttps://spectrabase.com/spectrum/Ec4bBEb5PeGtrans-2-Decenoic acid, methyl esterhttps://spectrabase.com/spectrum/8cLJ0KhcJkxtrans-2-Decenoic acid, methyl esterhttps://spectrabase.com/spectrum/EcP7C5sOsG
- Springer Nature
- Thieme ChemistryLICENSEThe Thieme Chemistry contribution within PubChem is provided under a CC-BY-NC-ND 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc-nd/4.0/
- Wikidata(E)-methyl 2-decenoatehttps://www.wikidata.org/wiki/Q27269022
- PubChem
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/NORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 389274177https://pubchem.ncbi.nlm.nih.gov/substance/389274177
CONTENTS