Linarin
PubChem CID
5317025
Molecular Formula
Synonyms
- Linarin
- 480-36-4
- Acaciin
- Buddleoside
- Linarine
Molecular Weight
592.5 g/mol
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Dates
- Create:2005-08-09
- Modify:2025-01-18
Description
Linarin has been reported in Silene firma, Pinus sylvestris, and other organisms with data available.
Chemical Structure Depiction
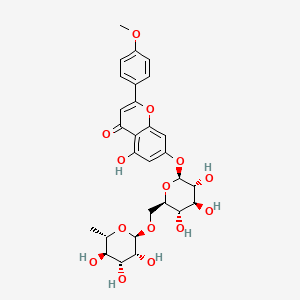
5-hydroxy-2-(4-methoxyphenyl)-7-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-[[(2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxymethyl]oxan-2-yl]oxychromen-4-one
Computed by Lexichem TK 2.7.0 (PubChem release 2021.10.14)
InChI=1S/C28H32O14/c1-11-21(31)23(33)25(35)27(39-11)38-10-19-22(32)24(34)26(36)28(42-19)40-14-7-15(29)20-16(30)9-17(41-18(20)8-14)12-3-5-13(37-2)6-4-12/h3-9,11,19,21-29,31-36H,10H2,1-2H3/t11-,19+,21-,22+,23+,24-,25+,26+,27+,28+/m0/s1
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
YFVGIJBUXMQFOF-PJOVQGMDSA-N
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
C[C@H]1[C@@H]([C@H]([C@H]([C@@H](O1)OC[C@@H]2[C@H]([C@@H]([C@H]([C@@H](O2)OC3=CC(=C4C(=C3)OC(=CC4=O)C5=CC=C(C=C5)OC)O)O)O)O)O)O)O
Computed by OEChem 2.3.0 (PubChem release 2024.12.12)
C28H32O14
Computed by PubChem 2.2 (PubChem release 2021.10.14)
- acaciin
- linarin
- Linarin
- 480-36-4
- Acaciin
- Buddleoside
- Linarine
- Linarigenin glycoside
- Acacetin 7-O-rutinoside
- Acacetin-7-O-rutinoside
- UNII-HBH2I685IU
- HBH2I685IU
- Acacetin-7-O-.eta-D-rutinoside
- LINARIN [MI]
- EINECS 207-547-6
- 5-hydroxy-2-(4-methoxyphenyl)-7-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-[[(2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxymethyl]oxan-2-yl]oxychromen-4-one
- Acacetin 7-rutinoside
- LINARIGENIN-GLUCOSIDE
- 5-Hydroxy-4'-methoxyflavone-7-O-rutinoside
- DTXSID40197382
- ACACETIN-.BETA.-RUTINOSIDE
- Acacetin-7-O-(6''-O-rhamnose)-beta-D-glucoside
- Acacetin 7-O-alpha-L-rhamnopyranosyl-(1->6)-beta-D-glucopyranoside
- 5-Hydroxy-2-(4-methoxyphenyl)-7-(((2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-((((2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)methyl)tetrahydro-2H-pyran-2-yl)oxy)-4H-chromen-4-one
- 7-((6-O-(6-Deoxy-alpha-L-mannopyranosyl)-beta-D-glucopyranosyl)oxy)-5-hydroxy-2-(4-methoxyphenyl)-4H-benzopyran-4-one
- 5,7-DIHYDROXY-4-METHOXYFLAVONE-D-GLUCOSIDO-L-RHAMNOSIDE
- Buddleoside;Linarine
- 4H-1-BENZOPYRAN-4-ONE, 7-((6-O-(6-DEOXY-.ALPHA.-L-MANNOPYRANOSYL)-.BETA.-D-GLUCOPYRANOSYL)OXY)-5-HYDROXY-2-(4-METHOXYPHENYL)-
- 4H-1-Benzopyran-4-one, 7-[[6-O-(6-deoxy-.alpha.-L-mannopyranosyl)-.beta.-D-glucopyranosyl]oxy]-5-hydroxy-2-(4-methoxyphenyl)-
- 7-((6-O-(6-DEOXY-.ALPHA.-L-MANNOPYRANOSYL)-.BETA.-D-GLUCOPYRANOSYL)OXY)-5-HYDROXY-2-(4-METHOXYPHENYL)-4H-1-BENZOPYRAN-4-ONE
- acacetin-beta-rutinoside
- Buddleoside,(S)
- Linarin;Buddleoside
- 5-hydroxy-2-(4-methoxyphenyl)-7-((2S,4R,5S)-3,4,5-trihydroxy-6-(((2R,4R,5R)-3,4,5-trihydroxy-6-methyloxan-2-yl)oxymethyl)oxan-2-yl)oxychromen-4-one
- 5-hydroxy-2-(4-methoxyphenyl)-7-((2S,4S,5S)-3,4,5-trihydroxy-6-(((2R,4S,5R)-3,4,5-trihydroxy-6-methyloxan-2-yl)oxymethyl)oxan-2-yl)oxychromen-4-one
- 5-hydroxy-2-(4-methoxyphenyl)-7-[(2S,4R,5S)-3,4,5-trihydroxy-6-[[(2R,4R,5R)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxymethyl]oxan-2-yl]oxychromen-4-one
- 5-hydroxy-2-(4-methoxyphenyl)-7-[(2S,4S,5S)-3,4,5-trihydroxy-6-[[(2R,4S,5R)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxymethyl]oxan-2-yl]oxychromen-4-one
- 7-((6-O-(6-DEOXY-ALPHA-L-MANNOPYRANOSYL)-BETA-D-GLUCOPYRANOSYL)OXY)-5-HYDROXY-2-(4-METHOXYPHENYL)-4H-1-BENZOPYRAN-4-ONE
- 7-[[6-O-(6-Deoxy-alpha-L-mannopyranosyl)-beta-D-glucopyranosyl]oxy]-5-hydroxy-2-(4-methoxyphenyl)-4H-1-benzopyran-4-one
- Linarin; 4H-1-Benzopyran-4-one, 7-[[6-O-(6-deoxy-a-L-mannopyranosyl)-ss-D-glucopyranosyl]oxy]-5-hydroxy-2-(4-methoxyphenyl)-; Linarin (6CI,7CI,8CI); 5-Hydroxy-4'-methoxyflavone-7-O-rutinoside; 7-[[6-O-(6-Deoxy-a-L-mannopyranosyl)-ss-D-glucopyranosyl]oxy]-5-
- MFCD00151178
- |A-HCH
- CHEMBL509502
- SCHEMBL1155704
- DTXCID50119873
- HMS3887E11
- HY-N0528
- BDBM50524083
- s9290
- STL564542
- AKOS015896769
- AC-8009
- CCG-270166
- CS-0009062
- NS00031751
- Q-100519
- Q1775837
- 4H-1-Benzopyran-4-one, 7-((6-O-(6-deoxy-alpha-L-mannopyranosyl)-beta-D-glucopyranosyl)oxy)-5-hydroxy-2-(4-methoxyphenyl)-
- 5-hydroxy-2-(4-methoxyphenyl)-4-oxo-4H-chromen-7-yl 6-O-(6-deoxy-alpha-L-mannopyranosyl)-beta-D-glucopyranoside
- 5-hydroxy-2-(4-methoxyphenyl)-7-((2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(((2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyltetrahydro-2H-pyran-2-yloxy)methyl)tetrahydro-2H-pyran-2-yloxy)-4H-chromen-4-one
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
592.5 g/mol
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
XLogP3-AA
Property Value
-0.5
Reference
Computed by XLogP3 3.0 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Donor Count
Property Value
7
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Acceptor Count
Property Value
14
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Rotatable Bond Count
Property Value
7
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Exact Mass
Property Value
592.17920569 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Monoisotopic Mass
Property Value
592.17920569 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Topological Polar Surface Area
Property Value
214 Ų
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Heavy Atom Count
Property Value
42
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
955
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
10
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
1
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2021.10.14)
Polyketides [PK] -> Flavonoids [PK12] -> Flavones and Flavonols [PK1211]
NIST Number
1155015
Instrument Type
IT/ion trap
Collision Energy
0
Spectrum Type
MS2
Precursor Type
[M+H]+
Precursor m/z
593.1865
Total Peaks
7
m/z Top Peak
447.1
m/z 2nd Highest
285
m/z 3rd Highest
445.9
Thumbnail
NIST Number
1155044
Instrument Type
IT/ion trap
Collision Energy
0
Spectrum Type
MS2
Precursor Type
[M-H]-
Precursor m/z
591.1719
Total Peaks
5
m/z Top Peak
283
m/z 2nd Highest
268
m/z 3rd Highest
419.3
Thumbnail
MoNA ID
MS Category
Experimental
MS Type
LC-MS
MS Level
MS1
Instrument
LCMS-IT-TOF
Instrument Type
LC-ESI-ITTOF
Ionization Mode
positive
Retention Time
14.101667 min
Top 5 Peaks
593.194200 100
594.201100 34.41
447.130900 17.10
285.072400 9.98
595.185300 9.18
License
CC BY-SA
MoNA ID
MS Category
Experimental
MS Type
LC-MS
MS Level
MS2
Precursor Type
[M+H]+
Precursor m/z
593.1864822
Instrument
Waters Xevo G2 Q-Tof
Instrument Type
LC-ESI-QTOF
Ionization Mode
positive
Collision Energy
50 eV
Retention Time
5.192966
Top 5 Peaks
285.07596 100
286.0795 19.80
270.05515 12.30
242.05258 5.20
287.08673 2.80
MoNA ID
MS Category
Experimental
MS Type
Other
MS Level
MS2
Precursor Type
[M-H]-
Precursor m/z
591.54
Instrument
TQD, Waters
Instrument Type
Flow-injection QqQ/MS
Ionization
ESI
Ionization Mode
negative
Collision Energy
50
Top 5 Peaks
283 100
284 11.93
282 8.19
MoNA ID
MS Category
Experimental
MS Type
Other
MS Level
MS2
Precursor Type
[M-H]-
Precursor m/z
591.54
Instrument
TQD, Waters
Instrument Type
Flow-injection QqQ/MS
Ionization
ESI
Ionization Mode
negative
Collision Energy
40
Top 5 Peaks
283 100
282 22.95
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Same Connectivity Count
Same Parent, Connectivity Count
Same Parent, Exact Count
Mixtures, Components, and Neutralized Forms Count
Similar Compounds (2D)
Similar Conformers (3D)
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=YFVGIJBUXMQFOF-PJOVQGMDSA-N
The LOTUS Initiative for Open Natural Products Research: frozen dataset union wikidata (with metadata) | DOI:10.5281/zenodo.5794106
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/
- ChemIDplusChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA DSSToxCompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-notice7-[[6-O-(6-deoxy-α-L-mannopyranosyl)-β-D-glucopyranosyl]oxy]-5-hydroxy-2-(4-methoxyphenyl)-4H-benzopyran-4-onehttps://echa.europa.eu/substance-information/-/substanceinfo/100.006.862
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- Comparative Toxicogenomics Database (CTD)LICENSEIt is to be used only for research and educational purposes. Any reproduction or use for commercial purpose is prohibited without the prior express written permission of NC State University.http://ctdbase.org/about/legal.jsp
- Japan Chemical Substance Dictionary (Nikkaji)
- LIPID MAPSAcacetin 7-rutinosidehttps://lipidmaps.org/databases/lmsd/LMPK12110449Lipid Classificationhttps://www.lipidmaps.org/
- LOTUS - the natural products occurrence databaseLICENSEThe code for LOTUS is released under the GNU General Public License v3.0.https://lotus.nprod.net/LOTUS Treehttps://lotus.naturalproducts.net/
- Natural Product Activity and Species Source (NPASS)
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- Metabolomics WorkbenchAcacetin 7-rutinosidehttps://www.metabolomicsworkbench.org/data/StructureData.php?RegNo=23534
- NIST Mass Spectrometry Data CenterLICENSEData covered by the Standard Reference Data Act of 1968 as amended.https://www.nist.gov/srd/public-law
- SpectraBase
- Wikidata
- PubChem
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.html
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/NORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 396405218https://pubchem.ncbi.nlm.nih.gov/substance/396405218
CONTENTS