5,6-trans-Vitamin D3
PubChem CID
5283711
Molecular Formula
Synonyms
- 22350-41-0
- trans-Vitamin D3
- 5,6-trans-Vitamin D3
- (1S,3E)-3-[(2E)-2-[(1R,3aS,7aR)-7a-methyl-1-[(2R)-6-methylheptan-2-yl]-2,3,3a,5,6,7-hexahydro-1H-inden-4-ylidene]ethylidene]-4-methylidenecyclohexan-1-ol
- cholecalciferol EP impurity A
Molecular Weight
384.6 g/mol
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Dates
- Create:2006-01-13
- Modify:2025-01-18
Description
5,6-trans-vitamin D3 is a member of the class of D3 vitamins that is calciol in which the double bond at position 5 adopts a trans-configuration. During exposure to sunlight, previtamin D3 and vitamin D3 in the skin become photoisomerized to 5,6-trans-vitamin D3. It has a role as a human metabolite. It is a member of D3 vitamins, a seco-cholestane, a secondary alcohol and a hydroxy seco-steroid. It is functionally related to a calciol.
5,6-trans-Vitamin D3 has been reported in Solanum glaucophyllum and Homo sapiens with data available.
Chemical Structure Depiction
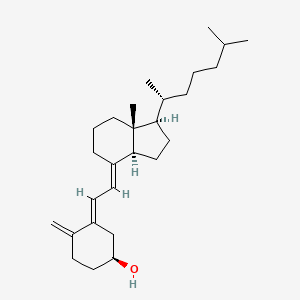
(1S,3E)-3-[(2E)-2-[(1R,3aS,7aR)-7a-methyl-1-[(2R)-6-methylheptan-2-yl]-2,3,3a,5,6,7-hexahydro-1H-inden-4-ylidene]ethylidene]-4-methylidenecyclohexan-1-ol
Computed by Lexichem TK 2.7.0 (PubChem release 2021.10.14)
InChI=1S/C27H44O/c1-19(2)8-6-9-21(4)25-15-16-26-22(10-7-17-27(25,26)5)12-13-23-18-24(28)14-11-20(23)3/h12-13,19,21,24-26,28H,3,6-11,14-18H2,1-2,4-5H3/b22-12+,23-13+/t21-,24+,25-,26+,27-/m1/s1
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
QYSXJUFSXHHAJI-FVUVGDFOSA-N
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
C[C@H](CCCC(C)C)[C@H]1CC[C@@H]\2[C@@]1(CCC/C2=C\C=C\3/C[C@H](CCC3=C)O)C
Computed by OEChem 2.3.0 (PubChem release 2024.12.12)
C27H44O
Computed by PubChem 2.2 (PubChem release 2021.10.14)
- 22350-41-0
- trans-Vitamin D3
- 5,6-trans-Vitamin D3
- (1S,3E)-3-[(2E)-2-[(1R,3aS,7aR)-7a-methyl-1-[(2R)-6-methylheptan-2-yl]-2,3,3a,5,6,7-hexahydro-1H-inden-4-ylidene]ethylidene]-4-methylidenecyclohexan-1-ol
- cholecalciferol EP impurity A
- (5E,7E)-(3S)-9,10-seco-5,7,10(19)-cholestatrien-3-ol
- 5,6-trans-Vitamin D3, ~90%
- (5E)-vitamin D3 / (5E)-cholecalciferol / (5E)-calciol
- (1S,3E)-3-{2-[(1R,3aS,4E,7aR)-7a-methyl-1-[(2R)-6-methylheptan-2-yl]-octahydro-1H-inden-4-ylidene]ethylidene}-4-methylidenecyclohexan-1-ol
- 5,6-trans-Cholecalciferol
- DTXCID306294
- CAS-67-97-0
- NCGC00159331-02
- (5E)-calciol
- (5e)-cholecalciferol
- (3S,5E,7E)-9,10-secocholesta-5,7,10-trien-3-ol
- (5E)-vitamin D3
- CHOLECALCIFEROL IMPURITY A [EP IMPURITY]
- Cholecalciferol, (5E)-
- SCHEMBL3127
- W28SLM7V6D
- CHEMBL1236645
- CHEBI:145213
- QYSXJUFSXHHAJI-FVUVGDFOSA-N
- DTXSID901316617
- Tox21_111578
- Tox21_202546
- HY-15398A
- LMST03020220
- AKOS026750034
- NCGC00159331-07
- NCGC00159331-15
- NCGC00179565-01
- NCGC00179565-02
- NCGC00260095-01
- AS-82639
- DA-70152
- CS-0111447
- NS00080863
- E85758
- EN300-22979549
- (E,E)-9,10-secocholesta-5,7,10(19)-trien-3beta-ol
- 9,10-Secocholesta-5,7,10(19)-trien-3beta-ol, (E,E)-
- (3beta,5E,7E)-9,10-secocholesta-5,7,10(19)-trien-3-ol
- 9,10-Secocholesta-5,7,10(19)-trien-3-ol, (3beta,5E,7E)-
- (1S,3E)-3-[(2E)-2-[(1R,3aS,7aR)-1-[(1R)-1,5-Dimethylhexyl]octahydro-7a-methyl-4H-inden-4-ylidene]ethylidene]-4-methylenecyclohexanol
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
384.6 g/mol
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
XLogP3-AA
Property Value
7.9
Reference
Computed by XLogP3 3.0 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Donor Count
Property Value
1
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Acceptor Count
Property Value
1
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Rotatable Bond Count
Property Value
6
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Exact Mass
Property Value
384.339216023 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Monoisotopic Mass
Property Value
384.339216023 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Topological Polar Surface Area
Property Value
20.2 Ų
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Heavy Atom Count
Property Value
28
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
610
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
5
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
2
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
1
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2021.10.14)
Lipids -> Sterol Lipids [ST] -> Secosteroids [ST03] -> Vitamin D3 and derivatives [ST0302]
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Same Connectivity Count
Same Stereo Count
Same Isotope Count
Same Parent, Connectivity Count
Same Parent, Stereo Count
Same Parent, Isotope Count
Same Parent, Exact Count
Mixtures, Components, and Neutralized Forms Count
Similar Compounds (2D)
Similar Conformers (3D)
Protein Structures Count
The LOTUS Initiative for Open Natural Products Research: frozen dataset union wikidata (with metadata) | DOI:10.5281/zenodo.5794106
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/trans-Vitamin D3https://commonchemistry.cas.org/detail?cas_rn=22350-41-0
- EPA DSSToxtrans-Vitamin D3https://comptox.epa.gov/dashboard/DTXSID901316617CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingCholecalciferol, (5E)-https://gsrs.ncats.nih.gov/ginas/app/beta/substances/W28SLM7V6D
- ChEBI5,6-trans-vitamin D3https://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:145213
- LOTUS - the natural products occurrence databaseLICENSEThe code for LOTUS is released under the GNU General Public License v3.0.https://lotus.nprod.net/5,6-trans-Vitamin D3https://www.wikidata.org/wiki/Q105230494LOTUS Treehttps://lotus.naturalproducts.net/
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- LIPID MAPS(5E)-vitamin D3https://lipidmaps.org/databases/lmsd/LMST03020220Lipid Classificationhttps://www.lipidmaps.org/
- Metabolomics Workbench(5E)-vitamin D3 / (5E)-cholecalciferol / (5E)-calciolhttps://www.metabolomicsworkbench.org/data/StructureData.php?RegNo=357695,6-trans-Vitamin D3https://www.metabolomicsworkbench.org/data/StructureData.php?RegNo=39007
- Rhea - Annotated Reactions DatabaseLICENSERhea has chosen to apply the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0/). This means that you are free to copy, distribute, display and make commercial use of the database in all legislations, provided you credit (cite) Rhea.https://www.rhea-db.org/help/license-disclaimer
- SpectraBaseCHOLECALCIFEROLhttps://spectrabase.com/spectrum/EyuIcn5P0g4
- Wikidatacholecalciferolhttps://www.wikidata.org/wiki/Q105230494
- Wiley
- PubChem
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/NORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
CONTENTS