Lycoxanthin
PubChem CID
5281245
Molecular Formula
Synonyms
- Lycoxanthin
- all-trans-Lycoxanthin
- Lycoxanthin [MI]
- Lycoxanthin, all-trans-
- Lycopen-16-ol, all-trans-
Molecular Weight
552.9 g/mol
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Dates
- Create:2005-03-27
- Modify:2025-01-18
Description
Lycoxanthin is a carotenol that is carotenol psi,psi-carotene substituted by a hydroxy group. It derives from a hydride of a lycopene.
Lycoxanthin has been reported in Nephroma arcticum, Equisetum arvense, and other organisms with data available.
Chemical Structure Depiction
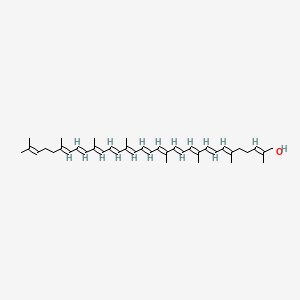
Conformer generation is disallowed since too flexible
(2E,6E,8E,10E,12E,14E,16E,18E,20E,22E,24E,26E)-2,6,10,14,19,23,27,31-octamethyldotriaconta-2,6,8,10,12,14,16,18,20,22,24,26,30-tridecaen-1-ol
Computed by Lexichem TK 2.7.0 (PubChem release 2021.10.14)
InChI=1S/C40H56O/c1-33(2)18-12-21-36(5)24-15-27-37(6)25-13-22-34(3)19-10-11-20-35(4)23-14-26-38(7)28-16-29-39(8)30-17-31-40(9)32-41/h10-11,13-16,18-20,22-29,31,41H,12,17,21,30,32H2,1-9H3/b11-10+,22-13+,23-14+,27-15+,28-16+,34-19+,35-20+,36-24+,37-25+,38-26+,39-29+,40-31+
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
IFTRFNLCKUZSNG-SFEKFZNLSA-N
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
CC(=CCC/C(=C/C=C/C(=C/C=C/C(=C/C=C/C=C(\C)/C=C/C=C(\C)/C=C/C=C(\C)/CC/C=C(\C)/CO)/C)/C)/C)C
Computed by OEChem 2.3.0 (PubChem release 2024.12.12)
C40H56O
Computed by PubChem 2.2 (PubChem release 2021.10.14)
19891-74-8
- Lycoxanthin
- all-trans-Lycoxanthin
- Lycoxanthin [MI]
- Lycoxanthin, all-trans-
- Lycopen-16-ol, all-trans-
- 19891-74-8
- Lycopen-16-ol
- omega,omega-Caroten-16-ol
- UNII-Z178H2XR4Q
- Z178H2XR4Q
- .psi.,.psi.-Caroten-16-ol
- psi,psi-caroten-1-ol
- CHEBI:6602
- DTXSID00415094
- (2E,6E,8E,10E,12E,14E,16E,18E,20E,22E,24E,26E)-2,6,10,14,19,23,27,31-octamethyldotriaconta-2,6,8,10,12,14,16,18,20,22,24,26,30-tridecaen-1-ol
- .OMEGA.,.OMEGA.-CAROTEN-16-OL
- psi,psi-Caroten-16-ol
- (1E)-Psi,psi-carotene #
- SCHEMBL115941
- DTXCID60365945
- LMPR01070276
- C08603
- Q27107265
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
552.9 g/mol
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
XLogP3-AA
Property Value
14.3
Reference
Computed by XLogP3 3.0 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Donor Count
Property Value
1
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Acceptor Count
Property Value
1
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Rotatable Bond Count
Property Value
17
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Exact Mass
Property Value
552.433116406 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Monoisotopic Mass
Property Value
552.433116406 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Topological Polar Surface Area
Property Value
20.2 Ų
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Heavy Atom Count
Property Value
41
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
1170
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
12
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
1
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2021.10.14)
Lipids -> Prenol Lipids [PR] -> Isoprenoids [PR01] -> C40 isoprenoids (tetraterpenes) [PR0107]
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Same Connectivity Count
Same Parent, Connectivity Count
Mixtures, Components, and Neutralized Forms Count
Similar Compounds (2D)
Similar Conformers (3D)
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=IFTRFNLCKUZSNG-SFEKFZNLSA-N
The LOTUS Initiative for Open Natural Products Research: frozen dataset union wikidata (with metadata) | DOI:10.5281/zenodo.5794106
- ChEBI
- LOTUS - the natural products occurrence databaseLICENSEThe code for LOTUS is released under the GNU General Public License v3.0.https://lotus.nprod.net/Lycoxanthinhttps://www.wikidata.org/wiki/Q27107265LOTUS Treehttps://lotus.naturalproducts.net/
- ChemIDplusChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA DSSToxCompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Japan Chemical Substance Dictionary (Nikkaji)
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.htmlPhytochemical compoundshttp://www.genome.jp/kegg-bin/get_htext?br08003.keg
- KNApSAcK Species-Metabolite Database
- Natural Product Activity and Species Source (NPASS)
- LIPID MAPSLipid Classificationhttps://www.lipidmaps.org/
- Metabolomics Workbench
- NIST Mass Spectrometry Data CenterLICENSEData covered by the Standard Reference Data Act of 1968 as amended.https://www.nist.gov/srd/public-lawLycoxanthinhttp://www.nist.gov/srd/nist1a.cfm
- SpectraBase.psi.,.psi.-Caroten-16-olhttps://spectrabase.com/spectrum/81iSL47aNFk
- Springer Nature
- Wikidatalycoxanthinhttps://www.wikidata.org/wiki/Q27107265
- PubChem
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 403065259https://pubchem.ncbi.nlm.nih.gov/substance/403065259
CONTENTS