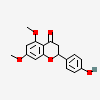
5,7-Dimethoxy-4'-hydroxyflavanone
PubChem CID
5271551
Molecular Formula
Synonyms
- 5,7-Dimethoxy-4'-hydroxyflavanone
- 26207-67-0
- 2-(4-hydroxyphenyl)-5,7-dimethoxychroman-4-one
- Naringenin 5,7-dimethyl ether
- CHEMBL2299039
Molecular Weight
300.30 g/mol
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Dates
- Create:2005-10-07
- Modify:2024-12-07
Description
5,7-Dimethoxy-4'-hydroxyflavanone has been reported in Capsicum annuum and Streptomyces avermitilis with data available.
Chemical Structure Depiction
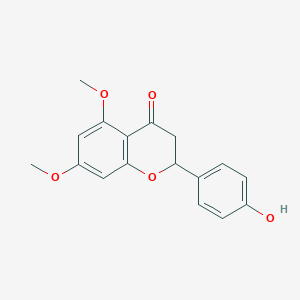
2-(4-hydroxyphenyl)-5,7-dimethoxy-2,3-dihydrochromen-4-one
Computed by LexiChem 2.6.6 (PubChem release 2019.06.18)
InChI=1S/C17H16O5/c1-20-12-7-15(21-2)17-13(19)9-14(22-16(17)8-12)10-3-5-11(18)6-4-10/h3-8,14,18H,9H2,1-2H3
Computed by InChI 1.0.5 (PubChem release 2019.06.18)
REBBZOCNEVVAPX-UHFFFAOYSA-N
Computed by InChI 1.0.5 (PubChem release 2019.06.18)
COC1=CC2=C(C(=O)CC(O2)C3=CC=C(C=C3)O)C(=C1)OC
Computed by OEChem 2.1.5 (PubChem release 2019.06.18)
C17H16O5
Computed by PubChem 2.1 (PubChem release 2019.06.18)
26207-67-0
- 5,7-Dimethoxy-4'-hydroxyflavanone
- 26207-67-0
- 2-(4-hydroxyphenyl)-5,7-dimethoxychroman-4-one
- Naringenin 5,7-dimethyl ether
- CHEMBL2299039
- 2-(4-hydroxyphenyl)-5,7-dimethoxy-3,4-dihydro-2H-1-benzopyran-4-one
- 4H-1-Benzopyran-4-one, 2,3-dihydro-2-(4-hydroxyphenyl)-5,7-dimethoxy-
- SCHEMBL5085603
- DTXSID40414920
- REBBZOCNEVVAPX-UHFFFAOYSA-N
- 4'-hydroxy-5,7-dimethoxyflavanone
- 5,7-dimethoxy-4'-hydroxy flavanone
- BDBM50488657
- LMPK12140595
- AKOS024285574
- DB-046892
- 5,7-dimethoxy-4'-hydroxyflavanone, AldrichCPR
- 2-(4-hydroxyphenyl)-5,7-dimethoxy-chroman-4-one
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
300.30 g/mol
Reference
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Property Name
XLogP3-AA
Property Value
2.5
Reference
Computed by XLogP3 3.0 (PubChem release 2019.06.18)
Property Name
Hydrogen Bond Donor Count
Property Value
1
Reference
Computed by Cactvs 3.4.6.11 (PubChem release 2019.06.18)
Property Name
Hydrogen Bond Acceptor Count
Property Value
5
Reference
Computed by Cactvs 3.4.6.11 (PubChem release 2019.06.18)
Property Name
Rotatable Bond Count
Property Value
3
Reference
Computed by Cactvs 3.4.6.11 (PubChem release 2019.06.18)
Property Name
Exact Mass
Property Value
300.09977361 Da
Reference
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Property Name
Monoisotopic Mass
Property Value
300.09977361 Da
Reference
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Property Name
Topological Polar Surface Area
Property Value
65Ų
Reference
Computed by Cactvs 3.4.6.11 (PubChem release 2019.06.18)
Property Name
Heavy Atom Count
Property Value
22
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
391
Reference
Computed by Cactvs 3.4.6.11 (PubChem release 2019.06.18)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
1
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
1
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2019.01.04)
Polyketides [PK] -> Flavonoids [PK12] -> Flavanones [PK1214]
NIST Number
1159257
Instrument Type
IT/ion trap
Collision Energy
0
Spectrum Type
MS2
Precursor Type
[M+H]+
Precursor m/z
301.1071
Total Peaks
9
m/z Top Peak
181
m/z 2nd Highest
147
m/z 3rd Highest
259.1
Thumbnail
NIST Number
1159275
Instrument Type
IT/ion trap
Collision Energy
0
Spectrum Type
MS2
Precursor Type
[M-H]-
Precursor m/z
299.0925
Total Peaks
11
m/z Top Peak
119
m/z 2nd Highest
255
m/z 3rd Highest
267
Thumbnail
Accession ID
Authors
Plant Biology, The Noble Foundation, Ardmore, OK, US/Dennis Fine, Daniel Wherritt, and Lloyd Sumner
Instrument
Bruker impact HD
Instrument Type
LC-ESI-QTOF
MS Level
MS
Ionization Mode
NEGATIVE
Ionization
ESI
Column Name
Waters Acquity BEH C18 1.7um x 2.1 x 150 mm
Retention Time
638.4 sec
Top 5 Peaks
299.0919 999
300.0957 160
345.0968 61
666.0217 39
301.0966 18
License
CC BY-NC-SA
Accession ID
Authors
Plant Biology, The Noble Foundation, Ardmore, OK, US/Dennis Fine, Daniel Wherritt, and Lloyd Sumner
Instrument
Bruker impact HD
Instrument Type
LC-ESI-QTOF
MS Level
MS2
Ionization Mode
NEGATIVE
Ionization
ESI
Collision Energy
20 eV
Column Name
Waters Acquity BEH C18 1.7um x 2.1 x 150 mm
Retention Time
638.4 sec
Precursor m/z
299.0899
Precursor Adduct
[M-H]-
Top 5 Peaks
119.0505 999
120.0561 68
299.0899 58
License
CC BY-NC-SA
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Same Connectivity Count
Same Parent, Connectivity Count
Similar Compounds (2D)
Similar Conformers (3D)
Same Count
PubMed Count
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=REBBZOCNEVVAPX-UHFFFAOYSA-N
The LOTUS Initiative for Open Natural Products Research: frozen dataset union wikidata (with metadata) | DOI:10.5281/zenodo.5794106
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- EPA DSSTox5,7-Dimethoxy-4'-hydroxyflavanonehttps://comptox.epa.gov/dashboard/DTXSID40414920CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- Japan Chemical Substance Dictionary (Nikkaji)
- KNApSAcK Species-Metabolite Database5,7-Dimethoxy-4'-hydroxyflavanonehttp://www.knapsackfamily.com/knapsack_core/info.php?sname=C_ID&word=C00061139
- LOTUS - the natural products occurrence databaseLICENSEThe code for LOTUS is released under the GNU General Public License v3.0.https://lotus.nprod.net/5,7-Dimethoxy-4'-hydroxyflavanonehttps://www.wikidata.org/wiki/Q82224006LOTUS Treehttps://lotus.naturalproducts.net/
- LIPID MAPSNaringenin 5,7-dimethyl etherhttps://lipidmaps.org/databases/lmsd/LMPK12140595Lipid Classificationhttps://www.lipidmaps.org/
- MassBank Europe5,7-Dimethoxy-4'-hydroxyflavanonehttps://massbank.eu/MassBank/Result.jsp?inchikey=REBBZOCNEVVAPX-UHFFFAOYSA-N
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- Metabolomics WorkbenchNaringenin 5,7-dimethyl etherhttps://www.metabolomicsworkbench.org/data/StructureData.php?RegNo=27746
- NIST Mass Spectrometry Data CenterLICENSEData covered by the Standard Reference Data Act of 1968 as amended.https://www.nist.gov/srd/public-law5,7-Dimethoxy-4'-hydroxyflavanonehttp://www.nist.gov/srd/nist1a.cfm
- SpectraBase5,7-Dimethoxy-4'-hydroxyflavanonehttps://spectrabase.com/spectrum/LD690J9rusN5,7-Dimethoxy-4'-hydroxyflavanonehttps://spectrabase.com/spectrum/KSde4qdXn0h
- Springer Nature
- Wikidata5,7-Dimethoxy-4'-hydroxyflavanonehttps://www.wikidata.org/wiki/Q82224006
- PubChem
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 389857992https://pubchem.ncbi.nlm.nih.gov/substance/389857992
CONTENTS