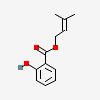
Prenyl salicylate
- Prenyl salicylate
- 68555-58-8
- 3-Methyl-2-butenyl salicylate
- 3-methylbut-2-enyl 2-hydroxybenzoate
- EINECS 271-434-8
- Create:2005-03-27
- Modify:2025-01-04
Not Classified
Reported as not meeting GHS hazard criteria by 236 of 239 companies (only 1.3% companies provided GHS information). For more detailed information, please visit ECHA C&L website.
Aggregated GHS information provided per 239 reports by companies from 3 notifications to the ECHA C&L Inventory.
Reported as not meeting GHS hazard criteria per 236 of 239 reports by companies. For more detailed information, please visit ECHA C&L website.
There are 2 notifications provided by 3 of 239 reports by companies with hazard statement code(s).
Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown.
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=MCAYDAOGSVHGEE-UHFFFAOYSA-N
- Australian Industrial Chemicals Introduction Scheme (AICIS)Benzoic acid, 2-hydroxy-, 3-methyl-2-butenyl esterhttps://services.industrialchemicals.gov.au/search-inventory/
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/Prenyl salicylatehttps://commonchemistry.cas.org/detail?cas_rn=68555-58-8
- ChemIDplusBenzoic acid, 2-hydroxy-, 3-methyl-2-buten-1-yl esterhttps://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0068555588ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA Chemicals under the TSCABenzoic acid, 2-hydroxy-, 3-methyl-2-buten-1-yl esterhttps://www.epa.gov/chemicals-under-tscaEPA TSCA Classificationhttps://www.epa.gov/tsca-inventory
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-notice3-methyl-2-butenyl salicylatehttps://chem.echa.europa.eu/100.064.9213-methyl-2-butenyl salicylate (EC: 271-434-8)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/59744
- International Fragrance Association (IFRA)LICENSE(c) The International Fragrance Association, 2007-2021. All rights reserved.https://ifrafragrance.org/links/copyright3-Methyl-2-butenyl salicylatehttps://ifrafragrance.org/priorities/ingredients/ifra-transparency-list
- New Zealand Environmental Protection Authority (EPA)LICENSEThis work is licensed under the Creative Commons Attribution-ShareAlike 4.0 International licence.https://www.epa.govt.nz/about-this-site/general-copyright-statement/
- EU Food Improvement AgentsPrenyl salicylatehttps://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32012R0872
- Japan Chemical Substance Dictionary (Nikkaji)
- NIST Mass Spectrometry Data CenterLICENSEData covered by the Standard Reference Data Act of 1968 as amended.https://www.nist.gov/srd/public-lawBenzoic acid, 2-hydroxy-, 3-methyl-2-butenyl esterhttp://www.nist.gov/srd/nist1a.cfm
- SpectraBasePrenyl salicylatehttps://spectrabase.com/spectrum/AorHpUwFcM5Prenyl salicylatehttps://spectrabase.com/spectrum/DG8gQSVyF2w
- Thieme ChemistryLICENSEThe Thieme Chemistry contribution within PubChem is provided under a CC-BY-NC-ND 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc-nd/4.0/
- WikidataPrenyl salicylatehttps://www.wikidata.org/wiki/Q126615186
- PubChem
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.html3-methyl-2-butenyl salicylatehttps://www.ncbi.nlm.nih.gov/mesh/67526487
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/NORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- EPA Substance Registry ServicesEPA SRS List Classificationhttps://sor.epa.gov/sor_internet/registry/substreg/LandingPage.do
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 390711184https://pubchem.ncbi.nlm.nih.gov/substance/390711184