Carubicin
PubChem CID
443831
Molecular Formula
Synonyms
- Carubicin
- Carminomycin
- 50935-04-1
- Carminomicin I
- 39472-31-6
Molecular Weight
513.5 g/mol
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Dates
- Create:2005-06-24
- Modify:2025-01-04
Description
Carminomycin is a toxic anthracycline antibiotic that is produced by Actinomadura carminata and also has potent antineoplastic activity. It has a role as an antineoplastic agent and an apoptosis inducer. It is an anthracycline antibiotic, an aminoglycoside antibiotic, a member of tetracenequinones, a member of p-quinones and a tertiary alpha-hydroxy ketone. It is a conjugate base of a carminomycin(1+). It derives from a hydride of a tetracene.
Carubicin is an anthracycline antineoplastic antibiotic isolated from the bacterium Actinomadura carminata. Carubicin intercalates into DNA and interacts with topoisomerase II, thereby inhibiting DNA replication and repair and RNA and protein synthesis.
A very toxic anthracycline-type antineoplastic related to DAUNORUBICIN, obtained from Actinomadura carminata.
Chemical Structure Depiction
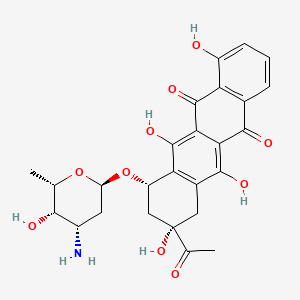
(7S,9S)-9-acetyl-7-[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy-4,6,9,11-tetrahydroxy-8,10-dihydro-7H-tetracene-5,12-dione
Computed by Lexichem TK 2.7.0 (PubChem release 2021.10.14)
InChI=1S/C26H27NO10/c1-9-21(30)13(27)6-16(36-9)37-15-8-26(35,10(2)28)7-12-18(15)25(34)20-19(23(12)32)22(31)11-4-3-5-14(29)17(11)24(20)33/h3-5,9,13,15-16,21,29-30,32,34-35H,6-8,27H2,1-2H3/t9-,13-,15-,16-,21+,26-/m0/s1
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
XREUEWVEMYWFFA-CSKJXFQVSA-N
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
C[C@H]1[C@H]([C@H](C[C@@H](O1)O[C@H]2C[C@@](CC3=C2C(=C4C(=C3O)C(=O)C5=C(C4=O)C(=CC=C5)O)O)(C(=O)C)O)N)O
Computed by OEChem 2.3.0 (PubChem release 2024.12.12)
C26H27NO10
Computed by PubChem 2.2 (PubChem release 2021.10.14)
52794-97-5 (hydrochloride)
- Carminomicin
- Carminomycin
- Carminomycin I
- Carminomycin II
- Carminomycin III
- Carubicin
- Carubicin Hydrochloride
- Demethyldaunomycin
- Demethyldaunorubicin
- Hydrochloride, Carubicin
- Karminomicin
- Karminomycin
- NSC 180,024
- NSC 180024
- NSC-180,024
- NSC-180024
- NSC180,024
- NSC180024
- Rubeomycin A
- Rubeomycin A1
- Carubicin
- Carminomycin
- 50935-04-1
- Carminomicin I
- 39472-31-6
- Carminomycin I
- KARMINOMYCIN
- Carubicin [INN]
- Carubicinum
- Carubicina
- Carubicine
- O-Demethyldaunomycin
- Karminomitsin
- CHEBI:31359
- Carubicine [INN-French]
- Carubicinum [INN-Latin]
- Carubicina [INN-Spanish]
- CCRIS 961
- NSC-180024
- CCRIS 6185
- DTXSID3022742
- UNII-E7437K3983
- CARUBICIN [MI]
- NCGC00159344-02
- DTXCID602742
- (7S,9S)-9-acetyl-7-[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy-4,6,9,11-tetrahydroxy-8,10-dihydro-7H-tetracene-5,12-dione
- E7437K3983
- 50935-04-1 (free base)
- Carubicine (INN-French)
- Carubicinum (INN-Latin)
- Carubicina (INN-Spanish)
- (1S,3S)-3-Acetyl-1,2,3,4,6,11-hexahydro-3,5,10,12-tetrahydroxy-6,11-dioxo-1-naphthacenyl-3-amino-2,3,6-tridesoxy-alpha-L-lyxo-hexopyranosid
- (1S,3S)-3-acetyl-3,5,10,12-tetrahydroxy-6,11-dioxo-1,2,3,4,6,11-hexahydrotetracen-1-yl 3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranoside
- (7S,9S)-9-acetyl-7-[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyl-tetrahydropyran-2-yl]oxy-4,6,9,11-tetrahydroxy-8,10-dihydro-7H-tetracene-5,12-dione
- 5,12-Naphthacenedione,8-acetyl-10-[(3-amino-2,3,6-trideoxy-a-L-lyxo-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-1,6,8,11-tetrahydroxy-, (8S,10S)-
- (1S,3S)-3-ACETYL-1,2,3,4,6,11-HEXAHYDRO-3,5,10,12-TETRAHYDROXY-6,11-DIOXO-1-NAPHTHACENYL 3-AMINO-2,3,6-TRIDEOXY-.ALPHA.-L-LYXO-HEXOPYRANOSIDE
- (8S,10S)-8-acetyl-10-(((2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)-1,6,8,11-tetrahydroxy-7,8,9,10-tetrahydrotetracene-5,12-dione
- 5,12-NAPHTHACENEDIONE, 8-ACETYL-10-((3-AMINO-2,3,6-TRIDEOXY-.ALPHA.-L-LYXO-HEXOPYRANOSYL)OXY)-7,8,9,10-TETRAHYDRO-1,6,8,11-TETRAHYDROXY-, (8S-CIS)-
- 5,12-Naphthacenedione, 8-acetyl-10-((3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranosyl)oxy)-7,8,9,10-tetrahydro-1,6,8,11-tetrahydroxy-, (8S-cis)-
- CAS-50935-04-1
- Carubicin?
- NCGC00160675-01
- (7S,9S)-9-acetyl-7-((2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyl-tetrahydropyran-2-yl)oxy-4,6,9,11-tetrahydroxy-8,10-dihydro-7H-tetracene-5,12-dione
- (8S-cis)-acetyl-10-((3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranosyl)oxy)-7,8,9,10-tetrahydro-1,6,8,11-tetrahydroxy-5,12-naphthacenedione
- (8S-cis)-acetyl-10-[(3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-1,6,8,11-tetrahydroxy-5,12-naphthacenedione
- 5,12-naphthacenedione, 8-acetyl-10-((3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranosyl)oxy)-7,8,9,10-tetrahydro-1,6,8,11-tetra-hydroxy-(8S-cis)-(8CI 9CI)
- 5,12-naphthacenedione, 8-acetyl-10-[(3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-1,6,8,11-tetra-hydroxy-(8S-cis)-(8CI 9CI)
- Carminomycin; Carubicin
- 4-O-demethyldaunorubicin
- Antibiotic R 588A; Carminomicin I; Carminomycin I
- DSSTox_CID_2742
- SCHEMBL9552
- DSSTox_RID_76711
- DSSTox_GSID_22742
- CHEMBL474260
- EX-A2244
- HY-B2171
- Tox21_111589
- Tox21_111978
- BDBM50103635
- AKOS040744813
- NSC-180,024
- CS-0021097
- NS00011901
- Carminomycin, O-Demethyldaunomycin, CCRIS 961
- Q5047474
- (1S,3S)-3-ACETYL-1,2,3,4,6,11-HEXAHYDRO-3,5,10,12-TETRAHYDROXY-6,11-DIOXO-1-NAPHTHACENYL 3-AMINO-2,3,6-TRIDEOXY-ALPHA-L-LYXO-HEXOPYRANOSIDE
- (2S,3S)-3-acetyl-1,2,3,4,6,11-hexahydro-3,5,10,12-tetrahydroxy-6,11-dioxo-1-naphthacenyl 3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranoside
- 5,12-Naphthacenedione, 8-acetyl-10-((3-amino-2,3, 6-trideoxy-alpha-L-lyxo-hexopyranosyl)oxy)-7,8,9, 10-tetrahydro-1,6,8,11-tetra-hydroxy-(8S-cis)-(8CI 9CI)
- 5,12-naphthacenedione, 8-acetyl-10-((3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranosyl)oxy)-7,8,9,10-tetrahydro-1,6,8,11-tetra-hydroxy-,(8S-cis)-(8CI)-
- 5,12-Naphthacenedione, 8-acetyl-10-[(3-amino-2,3, 6-trideoxy-.alpha.-L-lyxo-hexopyranosyl)oxy]-7,8,9, 10-tetrahydro-1,6,8,11-tetra-hydroxy-(8S-cis)-(8CI 9CI)
- 5,12-Naphthacenedione, 8-acetyl-10-[(3-amino-2,3,6-trideoxy-.alpha.-L-lyxo-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-1,6,8,11-tetrahydroxy-, (8S,10S)-
- 5,12-naphthacenedione, 8-acetyl-10-[(3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-1,6,8,11-tetra-hydroxy-,(8S-cis)-(8CI)-(9CI)
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
513.5 g/mol
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
XLogP3
Property Value
1.5
Reference
Computed by XLogP3 3.0 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Donor Count
Property Value
6
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Acceptor Count
Property Value
11
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Rotatable Bond Count
Property Value
3
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Exact Mass
Property Value
513.16349606 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Monoisotopic Mass
Property Value
513.16349606 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Topological Polar Surface Area
Property Value
197 Ų
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Heavy Atom Count
Property Value
37
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
944
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
6
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
1
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2021.10.14)
Pharmaceuticals -> Antibiotics
S6 | ITNANTIBIOTIC | Antibiotic List from the ITN MSCA ANSWER | DOI:10.5281/zenodo.2621956
Accession ID
Authors
Nogawa T, Okano A, CSRS, RIKEN
Instrument
ABSciex API3200 LC/MS system
Instrument Type
LC-ESI-QQQ
MS Level
MS
Ionization Mode
POSITIVE
Precursor Adduct
[M+H]+
Top 5 Peaks
129.9 999
513.8 955
513.9 937
513.7 906
513.6 895
License
CC BY
Accession ID
Authors
Nogawa T, Okano A, CSRS, RIKEN
Instrument
Agilent 6410 Triple Quadrupole LC/MS system
Instrument Type
LC-ESI-QQQ
MS Level
MS2
Ionization Mode
POSITIVE
Precursor Adduct
[M+H]+
Top 5 Peaks
130.1 99
130 95
130.2 93
130.3 79
129.9 77
License
CC BY
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Same Connectivity Count
Same Parent, Connectivity Count
Same Parent, Exact Count
Mixtures, Components, and Neutralized Forms Count
Similar Compounds (2D)
Similar Conformers (3D)
PubMed Count
Antibiotics, Antineoplastic
Chemical substances, produced by microorganisms, inhibiting or preventing the proliferation of neoplasms. (See all compounds classified as Antibiotics, Antineoplastic.)
Topoisomerase II Inhibitors
Compounds that inhibit the activity of DNA TOPOISOMERASE II. Included in this category are a variety of ANTINEOPLASTIC AGENTS which target the eukaryotic form of topoisomerase II and ANTIBACTERIAL AGENTS which target the prokaryotic form of topoisomerase II. (See all compounds classified as Topoisomerase II Inhibitors.)
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=XREUEWVEMYWFFA-CSKJXFQVSA-N
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/
- ChemIDplusChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA DSSToxCompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- ChEBI
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- Drug Gene Interaction database (DGIdb)LICENSEThe data used in DGIdb is all open access and where possible made available as raw data dumps in the downloads section.http://www.dgidb.org/downloads
- Japan Chemical Substance Dictionary (Nikkaji)
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.html
- MassBank Europe
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- Metabolomics Workbench
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/CarminomycinNORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- Springer Nature
- Wikidata
- WikipediaMedroxyprogesterone acetatehttps://en.wikipedia.org/wiki/Medroxyprogesterone_acetate
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlAntibiotics, Antineoplastichttps://www.ncbi.nlm.nih.gov/mesh/68000903Topoisomerase II Inhibitorshttps://www.ncbi.nlm.nih.gov/mesh/68059005
- PubChem
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 403414609https://pubchem.ncbi.nlm.nih.gov/substance/403414609
CONTENTS