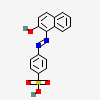
4-((2-Hydroxy-1-naphthyl)azo)benzenesulphonic acid
- 573-89-7
- Acid orange ii
- 4-((2-Hydroxy-1-naphthyl)azo)benzenesulphonic acid
- Mandarin G
- Tropaeolin OOO
- Create:2005-03-26
- Modify:2025-01-11
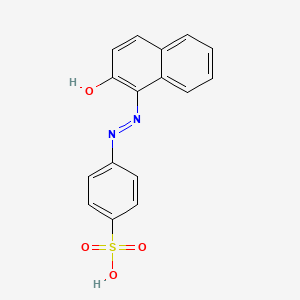
- 573-89-7
- Acid orange ii
- 4-((2-Hydroxy-1-naphthyl)azo)benzenesulphonic acid
- Mandarin G
- Tropaeolin OOO
- 4-[(2-hydroxy-1-naphthalenyl)azo]benzenesulfonic acid
- EINECS 209-362-6
- DFC Orange 4
- Acid orange 7 (free acid)
- SCHEMBL92162
- C.I. Acid Orange 7 parent
- CHEMBL2008747
- CHEMBL3306200
- DTXSID7047986
- SCHEMBL10053808
- SCHEMBL17460446
- C.I. 15510 Acid orange 7
- CHEBI:179961
- RDUJRVXKAIVTDH-UHFFFAOYSA-N
- 1-(4-Sulfophenylazo)-2-naphthol
- 1-(4'-sulfophenylazo)-2-naphthol
- NCI60_042199
- NS00010916
- benzenesulfonic acid, 4-(2-hydroxynaphth-1-ylazo)-
- 4-[(2-hydroxynaphthalen-1-yl)diazenyl]benzenesulfonic acid
- 4-[(2-hydroxynaphthalen-1-yl)diazenyl]benzenesulonic acid
329.0593 999
156.0444 890
128.0495 819
173.0142 480
312.0565 328
327.0444 999
170.9995 693
155.9886 167
93.0331 112
107.0365 75
Not Classified
Reported as not meeting GHS hazard criteria by 2 of 2 companies. For more detailed information, please visit ECHA C&L website.
Aggregated GHS information provided per 2 reports by companies from 1 notifications to the ECHA C&L Inventory.
Reported as not meeting GHS hazard criteria per 2 of 2 reports by companies. For more detailed information, please visit ECHA C&L website.
There are 0 notifications provided by 0 of 2 reports by companies with hazard statement code(s).
Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown.
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=RDUJRVXKAIVTDH-UHFFFAOYSA-N
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=RDUJRVXKAIVTDH-ISLYRVAYSA-N
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/1-(p-Sulfophenylazo)-2-naphtholhttps://commonchemistry.cas.org/detail?cas_rn=573-89-7
- ChemIDplus4-((2-Hydroxy-1-naphthyl)azo)benzenesulphonic acidhttps://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000573897ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA DSSToxC.I. Acid Orange 7 parenthttps://comptox.epa.gov/dashboard/DTXSID7047986CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-notice4-[(2-hydroxy-1-naphthyl)azo]benzenesulphonic acidhttps://echa.europa.eu/substance-information/-/substanceinfo/100.008.5124-[(2-hydroxy-1-naphthyl)azo]benzenesulphonic acid (EC: 209-362-6)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/126093
- ChEBI4-[(2-Hydroxy-1-naphthalenyl)azo]benzenesulfonic acidhttps://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:179961
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.html
- IUPAC Digitized pKa Datasetbenzenesulfonic acid, 4-(2-hydroxynaphth-1-ylazo)-https://github.com/IUPAC/Dissociation-Constants
- Japan Chemical Substance Dictionary (Nikkaji)
- MassBank EuropeAcid Orange 7 (free acid)https://massbank.eu/MassBank/Result.jsp?inchikey=RDUJRVXKAIVTDH-UHFFFAOYSA-N
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- Springer Nature
- WikidataC.I. Acid Orange 7 parenthttps://www.wikidata.org/wiki/Q81984473
- PubChem
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlAcid Orange IIhttps://www.ncbi.nlm.nih.gov/mesh/67456587
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/NORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 388667824https://pubchem.ncbi.nlm.nih.gov/substance/388667824SID 388705024https://pubchem.ncbi.nlm.nih.gov/substance/388705024