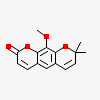
Luvangetin
PubChem CID
343582
Molecular Formula
Synonyms
- Luvangetin
- 483-92-1
- 10-methoxy-2,2-dimethylpyrano[3,2-g]chromen-8-one
- 10-Methoxy-8,8-dimethyl-2H,8H-benzo[1,2-b
- CHEBI:6586
Molecular Weight
258.27 g/mol
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Dates
- Create:2005-03-26
- Modify:2024-12-28
Description
Luvangetin is a member of coumarins.
Luvangetin has been reported in Zanthoxylum ailanthoides, Atalantia racemosa, and other organisms with data available.
Chemical Structure Depiction
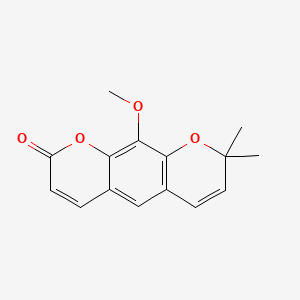
10-methoxy-2,2-dimethylpyrano[3,2-g]chromen-8-one
Computed by Lexichem TK 2.7.0 (PubChem release 2021.10.14)
InChI=1S/C15H14O4/c1-15(2)7-6-10-8-9-4-5-11(16)18-12(9)14(17-3)13(10)19-15/h4-8H,1-3H3
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
XYPWCJWXFYYGPA-UHFFFAOYSA-N
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
CC1(C=CC2=C(O1)C(=C3C(=C2)C=CC(=O)O3)OC)C
Computed by OEChem 2.3.0 (PubChem release 2021.10.14)
C15H14O4
Computed by PubChem 2.2 (PubChem release 2021.10.14)
- Luvangetin
- 483-92-1
- 10-methoxy-2,2-dimethylpyrano[3,2-g]chromen-8-one
- 10-Methoxy-8,8-dimethyl-2H,8H-benzo[1,2-b
- CHEBI:6586
- C09273
- 10-Methoxy-8,8-dimethyl-2H,8H-benzo[1,2-b:5,4-b']dipyran-2-one
- 10-METHOXY-8,8-DIMETHYL-2H,8H-PYRANO[3,2-G]CHROMEN-2-ONE
- 2H,8H-Benzo(1,2-b:5,4-b')dipyran-2-one, 10-methoxy-8,8-dimethyl-
- 10-Methoxy-8,8-dimethyl-2H,8H-benzo(1,2-b:5,4-b')dipyran-2-one
- AC1L7YNQ
- starbld0003698
- CHEMBL254379
- SCHEMBL14511557
- DTXSID60197485
- HY-N8340
- NSC383464
- AKOS040762006
- FS-6868
- NSC-383464
- DA-55082
- CS-0143314
- NS00097408
- E88907
- 10-methoxy-2,2-dimethyl-pyrano[3,2-g]chromen-8-one
- Q27107252
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
258.27 g/mol
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
XLogP3-AA
Property Value
2.8
Reference
Computed by XLogP3 3.0 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Donor Count
Property Value
0
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Acceptor Count
Property Value
4
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Rotatable Bond Count
Property Value
1
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Exact Mass
Property Value
258.08920892 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Monoisotopic Mass
Property Value
258.08920892 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Topological Polar Surface Area
Property Value
44.8Ų
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Heavy Atom Count
Property Value
19
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
437
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
1
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2021.10.14)
MoNA ID
MS Category
Experimental
MS Type
LC-MS
MS Level
MS2
Precursor Type
[M+H]+
Precursor m/z
259.0965
Instrument
Agilent 6530 Q-TOF
Instrument Type
LC-ESI-QTOF
Ionization Mode
positive
Collision Energy
40 V
Retention Time
5.1715
Top 5 Peaks
229.0492 100
128.062 81.62
243.0648 78.72
155.0494 56.59
183.0439 56.39
MoNA ID
MS Category
Experimental
MS Type
LC-MS
MS Level
MS2
Precursor Type
[M+H]+
Precursor m/z
259.0965
Instrument
Agilent 6530 Q-TOF
Instrument Type
LC-ESI-QTOF
Ionization Mode
positive
Collision Energy
20 V
Retention Time
5.1715
Top 5 Peaks
259.0965 100
244.0728 45.22
229.0493 32.49
243.0648 21.63
227.0701 5.46
MoNA ID
MS Category
Experimental
MS Type
Other
Precursor Type
[M+H]+
Top 5 Peaks
65.09709279833667 8.65
131.96105879833667 6.33
115.05909179833668 0.12
126.05230979833668 0.08
244.09572304180685 0.06
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Similar Compounds (2D)
Similar Conformers (3D)
Same Count
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=XYPWCJWXFYYGPA-UHFFFAOYSA-N
The LOTUS Initiative for Open Natural Products Research: frozen dataset union wikidata (with metadata) | DOI:10.5281/zenodo.5794106
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/
- ChemIDplus2H,8H-Benzo(1,2-b:5,4-b')dipyran-2-one, 10-methoxy-8,8-dimethyl-https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000483921ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA DSSTox2H,8H-Benzo(1,2-b:5,4-b')dipyran-2-one, 10-methoxy-8,8-dimethyl-https://comptox.epa.gov/dashboard/DTXSID60197485CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- ChEBI
- LOTUS - the natural products occurrence databaseLICENSEThe code for LOTUS is released under the GNU General Public License v3.0.https://lotus.nprod.net/Luvangetinhttps://www.wikidata.org/wiki/Q27107252LOTUS Treehttps://lotus.naturalproducts.net/
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.html
- DTP/NCILICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuse
- Japan Chemical Substance Dictionary (Nikkaji)
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.htmlPhytochemical compoundshttp://www.genome.jp/kegg-bin/get_htext?br08003.keg
- KNApSAcK Species-Metabolite Database
- Natural Product Activity and Species Source (NPASS)
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- Metabolomics Workbench
- SpectraBase
- Springer Nature
- Wikidataluvangetinhttps://www.wikidata.org/wiki/Q27107252
- PubChem
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/NORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 403333762https://pubchem.ncbi.nlm.nih.gov/substance/403333762
CONTENTS