Dihydrodehydroconiferyl alcohol, (7R,8S)-(-)-
PubChem CID
5274623
Molecular Formula
Synonyms
- 126253-41-6
- (2R,3S)-Dihydrodehydroconiferyl alcohol
- UNII-2B91L473ZL
- 7R,8S-Dihydrodehydrodiconiferyl alcohol
- Dihydrodehydroconiferyl alcohol, (7R,8S)-(-)-
Molecular Weight
360.4 g/mol
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Dates
- Create:2005-10-07
- Modify:2025-01-11
Description
(2R,3S)-dihydrodehydrodiconiferyl alcohol is a member of 1-benzofurans, a member of guaiacols, a guaiacyl lignin and a primary alcohol. It is an enantiomer of a (2S,3R)-dihydrodehydrodiconiferyl alcohol.
(2R,3S)-Dihydrodehydroconiferyl alcohol has been reported in Picea glehnii, Abies nephrolepis, and other organisms with data available.
See also: Acai fruit pulp (part of).
Chemical Structure Depiction
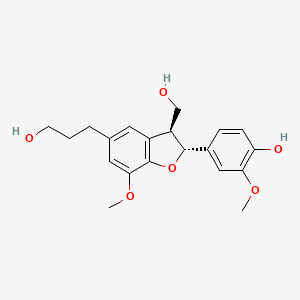
4-[(2R,3S)-3-(hydroxymethyl)-5-(3-hydroxypropyl)-7-methoxy-2,3-dihydro-1-benzofuran-2-yl]-2-methoxyphenol
Computed by Lexichem TK 2.7.0 (PubChem release 2021.10.14)
InChI=1S/C20H24O6/c1-24-17-10-13(5-6-16(17)23)19-15(11-22)14-8-12(4-3-7-21)9-18(25-2)20(14)26-19/h5-6,8-10,15,19,21-23H,3-4,7,11H2,1-2H3/t15-,19+/m1/s1
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
SBLZVJIHPWRSQQ-BEFAXECRSA-N
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
COC1=CC(=CC2=C1O[C@H]([C@@H]2CO)C3=CC(=C(C=C3)O)OC)CCCO
Computed by OEChem 2.3.0 (PubChem release 2024.12.12)
C20H24O6
Computed by PubChem 2.2 (PubChem release 2021.10.14)
126253-41-6
- dihydrodehydrodiconiferylalcohol
- lawsonicin
- pinonesinol
- 126253-41-6
- (2R,3S)-Dihydrodehydroconiferyl alcohol
- UNII-2B91L473ZL
- 7R,8S-Dihydrodehydrodiconiferyl alcohol
- Dihydrodehydroconiferyl alcohol, (7R,8S)-(-)-
- 5-Benzofuranpropanol, 2,3-dihydro-2-(4-hydroxy-3-methoxyphenyl)-3-(hydroxymethyl)-7-methoxy-, (2R,3S)-
- 2B91L473ZL
- (-)-(2R,3S)-Dihydrodehydroconiferyl alcohol
- (-)-(7R,8S)-Dihydrodehydroconiferyl alcohol
- dihydrodehydrodiconiferylalcohol
- 4-[(2R,3S)-3-(hydroxymethyl)-5-(3-hydroxypropyl)-7-methoxy-2,3-dihydro-1-benzofuran-2-yl]-2-methoxyphenol
- 28199-69-1
- (-)-(7R, 8S)-dihydrodehydrodiconiferyl alcohol
- 4-[(2R,3S)-3-(hydroxymethyl)-5-(3-hydroxypropyl)-7-methoxy-2,3-dihydrobenzofuran-2-yl]-2-methoxy-phenol
- (2R,3S)-Dihydrodehydrodiconiferyl alcohol
- (7R,8S)-Dihydrodehydrodiconiferyl alcohol
- CHEMBL253590
- Di-hydrodehydrodiconiferyl alcohol
- CHEBI:143258
- HY-N3745
- (7'R,8'S)-dihydrodehydrodiconifenyl alcohol 9'-o-b-dxylopyranoside
- AKOS032948731
- DA-62880
- FS-10632
- CS-0024144
- (7R,8S)-(-)-dihydrodehydroconiferyl alcohol
- (-)-(7R,8S)-Dihydrodehydrodiconiferyl alcohol
- G88952
- Q27254512
- (-)-5-Benzofuranpropanol, 2,3-dihydro-2-(4-hydroxy-3-methoxyphenyl)-3-(hydroxymethyl)-7-methoxy-, (2R,3S)-
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
360.4 g/mol
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
XLogP3-AA
Property Value
2.1
Reference
Computed by XLogP3 3.0 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Donor Count
Property Value
3
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Acceptor Count
Property Value
6
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Rotatable Bond Count
Property Value
7
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Exact Mass
Property Value
360.15728848 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Monoisotopic Mass
Property Value
360.15728848 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Topological Polar Surface Area
Property Value
88.4 Ų
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Heavy Atom Count
Property Value
26
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
433
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
2
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
1
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2021.10.14)
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Same Connectivity Count
Same Parent, Connectivity Count
Similar Compounds (2D)
Similar Conformers (3D)
Same Count
Acai fruit pulp (part of)
PubMed Count
The LOTUS Initiative for Open Natural Products Research: frozen dataset union wikidata (with metadata) | DOI:10.5281/zenodo.5794106
- ChEBI(2R,3S)-dihydrodehydrodiconiferyl alcoholhttps://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:143258
- LOTUS - the natural products occurrence databaseLICENSEThe code for LOTUS is released under the GNU General Public License v3.0.https://lotus.nprod.net/(2R,3S)-Dihydrodehydroconiferyl alcoholhttps://www.wikidata.org/wiki/Q27254512LOTUS Treehttps://lotus.naturalproducts.net/
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.html
- ChemIDplusDihydrodehydroconiferyl alcohol, (7R,8S)-(-)-https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0126253416ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingDIHYDRODEHYDROCONIFERYL ALCOHOL, (7R,8S)-(-)-https://gsrs.ncats.nih.gov/ginas/app/beta/substances/2B91L473ZL
- Japan Chemical Substance Dictionary (Nikkaji)
- Natural Product Activity and Species Source (NPASS)Dihydrodehydrodiconiferyl Alcoholhttps://bidd.group/NPASS/compound.php?compoundID=NPC29799
- Rhea - Annotated Reactions DatabaseLICENSERhea has chosen to apply the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0/). This means that you are free to copy, distribute, display and make commercial use of the database in all legislations, provided you credit (cite) Rhea.https://www.rhea-db.org/help/license-disclaimer
- SpectraBaseDIHYDRODEHYDRODICONIFERYLALCOHOLhttps://spectrabase.com/spectrum/7gPMFD7b2pTREL-(2-ALPHA,3-BETA)-7-O-METHYL-CEDRUSINhttps://spectrabase.com/spectrum/9iwstYd3Co7
- Wikidata(7R,8S)-(-)-dihydrodehydroconiferyl alcoholhttps://www.wikidata.org/wiki/Q27254512Dihydrodehydrodiconiferyl alcoholhttps://www.wikidata.org/wiki/Q72464814
- PubChem
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlpinonesinolhttps://www.ncbi.nlm.nih.gov/mesh/67489718
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
CONTENTS